In recent years, the field of kidney cancer has seen significant advancements, especially regarding treatment options for rarer subtypes beyond the more common clear cell renal cell carcinoma (ccRCC). Dr. Victor Grünwald, a medical oncologist specialising in genitourinary cancers at the University Hospital in Essen, Germany, shares insights into these emerging therapies, focusing on non-clear cell RCC (nccRCC), such as papillary RCC (pRCC), and other subentities. He also highlights the importance of the ESMO guidelines, which provide critical recommendations for treating rare RCC subtypes, while also emphasizing the increasing evidence supporting the effectiveness of combination therapies.
Clear cell carcinoma is the most common form of renal cell carcinoma, but other, rarer forms also arise in different regions of the kidney. These less common forms are often lumped under non-clear cell histologies, accounting for about 20–30% of all renal cell carcinomas and often have distinct clinical, pathological, and molecular features compared to ccRCC, and their diagnosis and treatment may vary accordingly. However, recent research emphasizes the need to recognize and treat these subtypes as distinct entities, as they require tailored therapeutic approaches.

© Dizman, N., Philip, E.J. & Pal, S.K.
Fig. 1. Location and histological subtypes of renal cell carcinoma (taken from Dizman, N et al).
Actionable Mutations in Rare Kidney Cancers
In recent years, the understanding of genetic mutations in kidney cancers has evolved. Actionable mutations—mutations that can be targeted with specific therapies—are not as prevalent in kidney cancer as once believed. However, certain mutations, such as the MET mutations, especially those found in pRCC, are relatively more common. These mutations have motivated increased focus on pRCC in clinical studies, leading to a more targeted and effective approach for some patient subgroups.
Cabozantinib and the Focus on Progression-Free Survival
The pivotal SWOG-1500 study assessed the efficacy of different single-agent therapies for pRCC. Among the tested treatments, cabozantinib, a tyrosine kinase inhibitor, emerged as the most effective, demonstrating a notable progression-free survival (PFS) of approximately six months. While overall survival rates did not show significant improvement, Dr. Grünwald underscored the importance of PFS as a primary endpoint due to study limitations.
Cabozantinib has also shown moderate efficacy in other rare translocation-associated RCC subtypes, as well as modest activity in Ductus Bellini patients, demonstrating a PFS of around 6 months, underscoring its potential utility as a single agent therapy in cases where histology-driven selection can optimize patient outcomes.
Can We Do Better For MET-Mutated Populations?
Can we provide more personalized treatments for patients with MET mutations or amplifications? In unselected patient populations, cabozantinib has outperformed other tyrosine kinase inhibitors. However, studies like the CREATE trial, which explored crizotinib, and the SAVIOR trial, which compared savolitinib to sunitinib in MET-mutated pRCC, demonstrated encouraging efficacy, showing that precision oncology is achievable and likely to become more common in the future. Dr. Grünwald emphasizes that single-agent treatments could be tailored to different subtypes and adds that using histology alone as an enrichment strategy is insufficient for optimal patient care.
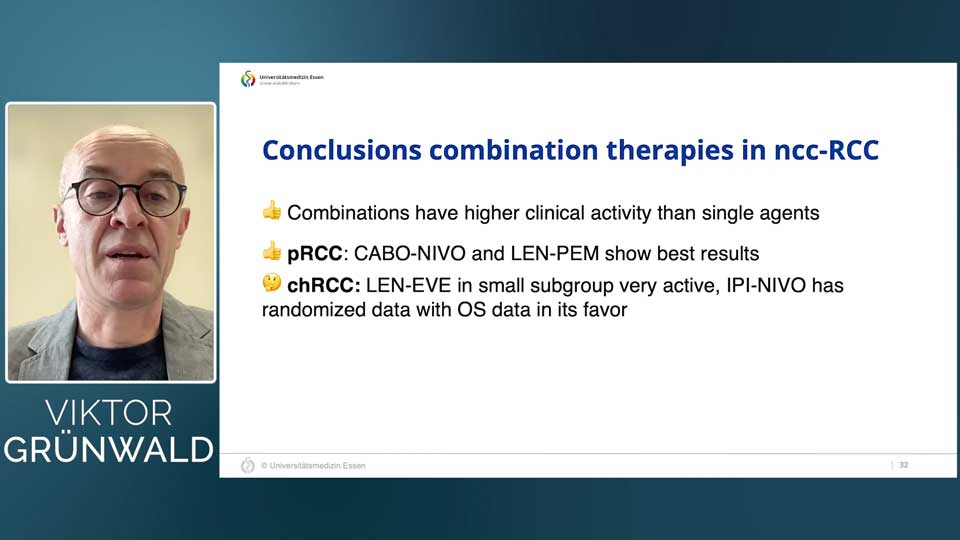
The Promise of Combination Therapies
The current ESMO guidelines provide treatment recommendations for rare kidney cancer subtypes, including advanced and metastatic nccRCC. While the use of single-agent cabozantinib for advanced or metastatic papillary renal cell carcinoma (pRCC) is important, Dr. Grünwald specifically points at the growing body of evidence supporting the clinical activity of combination therapies as opposed to single-agent cabozantinib as a preferred option for advanced or metastatic pRCC.
In the CA209-9KU study, the combination of cabozantinib and nivolumab demonstrated promising tumor shrinkage in pRCC patients. While the study size was small, having a total of only 47 patients, it provided a strong signal that combination approaches could benefit specific RCC subgroups.
Similarly, the HOPE221 study, which explored the combination of lenvatinib and everolimus, showed an objective response rate of 44% in a cohort of chromophobe RCC patients, which is remarkable given the rarity of this subtype and the limited efficacy of prior treatments. Further supporting lenvatinib’s potential, the KEYNOTE-B61 study investigated lenvatinib with pembrolizumab across various RCC subtypes, reinforcing the broad applicability of this combination.
The SUNNIFORECAST study further explored immune combinations by testing nivolumab and ipilimumab against standard care. This randomized phase two study reported a favorable one-year overall survival rate in patients with rare RCC subtypes with the combination approach over the standard of care, along with potential positive effects in the chromophobe subgroup, further supporting the use of immune combinations for rare histology subtypes of kidney cancers.
Immune Sensitivity and the Role of Specific Inhibitors
Dr. Grünwald emphasized that pRCC patients show a notable degree of immune sensitivity, which can guide the selection of immune combination treatments. Despite varying response rates across subtypes, the general consensus is that immune combinations, including combinations of cabozantinib with immune checkpoint inhibitors, should be offered where possible, particularly in pRCC. While evidence is still limited for rarer forms like chromophobe RCC, early indications are promising, though more research is needed.
Future Directions in Rare Kidney Cancer Treatment
In conclusion, Dr. Grünwald’s presentation underscored the need for precision oncology approaches in kidney cancer treatment, emphasizing histology-driven enrichment strategies and selective inhibitors with better tolerability and efficacy. While cabozantinib alone or in combination has set a promising standard, immune combinations represent a critical step forward for the RCC community, especially in treating rare and diverse histologies.
The future of kidney cancer treatment lies in refining combination strategies to address the needs of specific RCC subtypes, enhancing efficacy, and managing tolerability. As research progresses, these combination therapies are expected to continue reshaping the therapeutic landscape for patients with rare kidney cancers.
Watch the full expert talk by Dr. Viktor Grünwald to explore more on the treatment landscape for non-clear cell RCC entities.
References
N. Dizman, E. J. Philip, and S. K. Pal, “Genomic profiling in renal cell carcinoma,” Nature Reviews Nephrology, vol. 16, no. 8, pp. 435–451, Jun. 2020, doi: 10.1038/s41581-020-0301-x.
K. Attalla et al., “Prevalence and landscape of actionable genomic alterations in renal cell carcinoma,” Clinical Cancer Research, vol. 27, no. 20, pp. 5595–5606, Jul. 2021, doi: 10.1158/1078-0432.ccr-20-4058.
S. K. Pal et al., “A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial,” The Lancet, vol. 397, no. 10275, pp. 695–703, Feb. 2021, doi: 10.1016/s0140-6736(21)00152-5.
J. Thouvenin et al., “Efficacy of cabozantinib in advanced MiT family translocation renal cell carcinomas (TRCC).,” Journal of Clinical Oncology, vol. 39, no. 6_suppl, p. 274, Feb. 2021, doi: 10.1200/jco.2021.39.6_suppl.274.
G. Procopio et al., “A phase 2 prospective trial of cabozantinib as first-line treatment for metastatic collecting ducts renal cell carcinoma: The BONSAI trial (Meeturo 2) clinical trial information—NCT03354884.,” Journal of Clinical Oncology, vol. 39, no. 15_suppl, p. 4571, May 2021, doi: 10.1200/jco.2021.39.15_suppl.4571.
P. Schöffski et al., “Crizotinib achieves long-lasting disease control in advanced papillary renal-cell carcinoma type 1 patients with MET mutations or amplification. EORTC 90101 CREATE trial,” European Journal of Cancer, vol. 87, pp. 147–163, Nov. 2017, doi: 10.1016/j.ejca.2017.10.014.
T. K. Choueiri et al., “Efficacy of Savolitinib vs Sunitinib in Patients WithMET-Driven Papillary Renal Cell Carcinoma,” JAMA Oncology, vol. 6, no. 8, p. 1247, May 2020, doi: 10.1001/jamaoncol.2020.2218.
T. Powles et al., “Renal cell carcinoma: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up,” Annals of Oncology, vol. 35, no. 8, pp. 692–706, May 2024, doi: 10.1016/j.annonc.2024.05.537.
S. S. Tykodi et al., “Nivolumab plus ipilimumab in patients with advanced non-clear cell renal cell carcinoma (nccRCC): Safety and efficacy from CheckMate 920.,” Journal of Clinical Oncology, vol. 39, no. 6_suppl, p. 309, Feb. 2021, doi: 10.1200/jco.2021.39.6_suppl.309.
C.-H. Lee et al., “Phase II trial of cabozantinib plus nivolumab in patients with Non–Clear-Cell renal cell carcinoma and genomic correlates,” Journal of Clinical Oncology, vol. 40, no. 21, pp. 2333–2341, Mar. 2022, doi: 10.1200/jco.21.01944.
T. E. Hutson et al., “A phase II study of lenvatinib plus everolimus in patients with advanced non-clear cell renal cell carcinoma (nccRCC).,” Journal of Clinical Oncology, vol. 38, no. 6_suppl, p. 685, Feb. 2020, doi: 10.1200/jco.2020.38.6_suppl.685.
M. H. Voss et al., “First-line pembrolizumab plus lenvatinib for non–clear cell renal carcinomas (nccRCC): Extended follow-up of the phase 2 KEYNOTE-B61 study.,” Journal of Clinical Oncology, vol. 42, no. 4_suppl, p. 2, Jan. 2024, doi: 10.1200/jco.2024.42.4_suppl.2.
L. Bergmann et al., “LBA75 Prospective randomised phase-II trial of ipilimumab/nivolumab versus standard of care in non-clear cell renal cell cancer: Results of the SUNNIFORECAST trial,” Annals of Oncology, vol. 35, p. S1263, Sep. 2024, doi: 10.1016/j.annonc.2024.08.2318.