Clinically relevant HER2 expression is found in some gastrointestinal tumors, such as carcinomas of the stomach and colon, which makes therapeutic targeting with proven and new substances possible.
In the group of predictive markers evaluated in gastric cancer and carcinoma of the gastroesophageal junction (GEJ), HER2 overexpression now has the longest history. In HER2-positive advanced gastric and GEJ carcinoma, the HER2 inhibitor trastuzumab in combination with chemotherapy has been an established therapeutic pillar for more than ten years. In addition to inhibiting HER2-dependent signaling, trastuzumab works by activating immune effector cells (1). Additionally, this therapy also leads to the internalization and degradation of HER2 receptors.
However, challenges arise due to the pronounced intratumoral heterogeneity of HER2 expression, especially in gastric cancer (2). Over time, HER2-targeted treatment can lead to HER2 loss and the development of resistance aberrations, such as FGFR2 and CCNE1 amplifications (3). A Germany-wide network study confirmed the frequency of heterogeneous HER2 expression (4). It also showed that a clear clinical benefit from trastuzumab can only be expected with HER2 expression on the cell surface of at least 40% of the cells. In this group, overall survival (OS) clearly exceeded that with lower HER2 expression (Fig.).
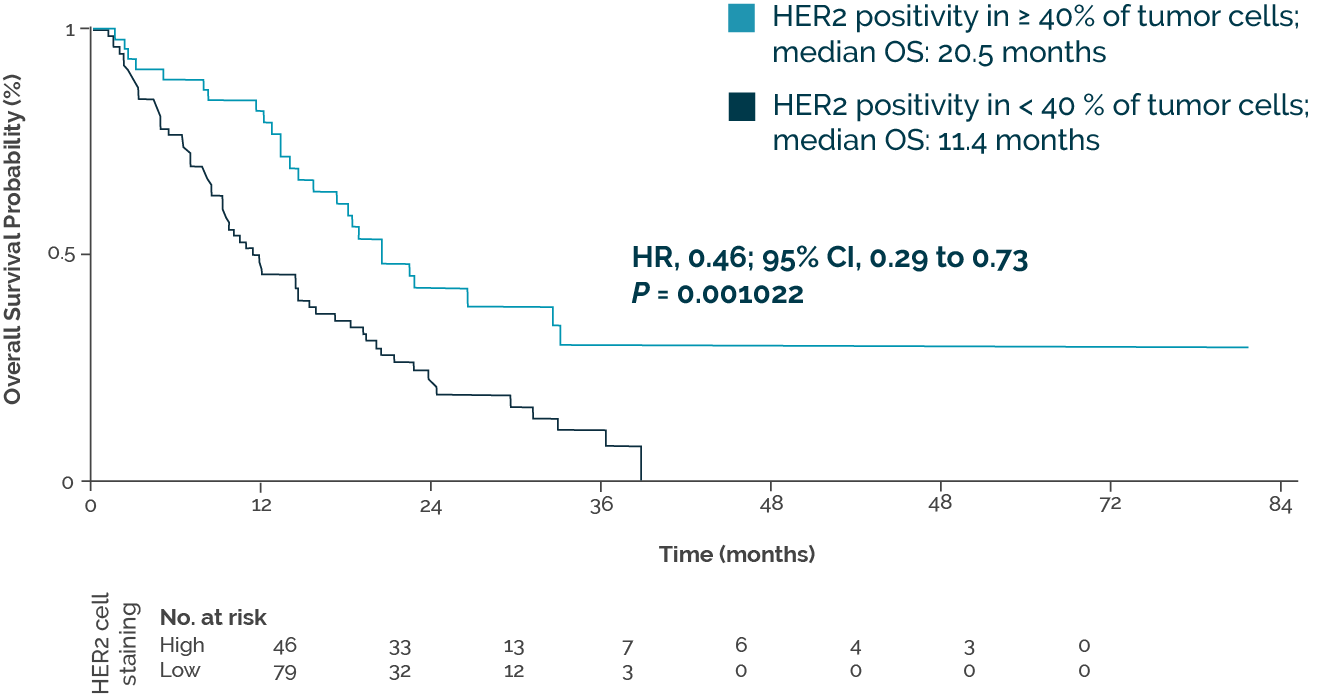
© OncoViews
Optimized threshold for differentiating between patients receiving trastuzumab treatment based on HER2+ tumor cell staining by immunohistochemistry. Adapted from Haffner I et al.
T-DXd & Zanidatamab
These challenges can be at least partially overcome with the antibody-drug conjugate trastuzumab deruxtecan (T-DXd), which was approved for HER2-positive gastric and GEJ carcinoma following the failure of trastuzumab. After internalization in HER2-overexpressing cells, the highly cytotoxic topoisomerase I inhibitor deruxtecan exerts its effects not only within the HER2-positive cell but also in the immediate environment (bystander effect) (5).
T-DXd showed superior efficacy compared to irinotecan or paclitaxel in the Asian DESTINY-Gastric01 study in the third-line setting in terms of overall survival (OS: 12.5 vs. 8.4 months; HR: 0.59; p = 0.01) and overall response rate (ORR: 43% vs. 12%) (6). The single-arm DESTINY-Gastric02 study also showed an ORR of >40% under second-line T-DXd in a Western population, which is remarkable in this setting (7). The median OS was 12.1 months. There was also high consistency between the two studies in terms of side effects, with potentially lethal pneumonitis requiring the greatest attention. It must be kept in mind that patients undergoing T-DXd therapy can develop pneumonitis at any time.
The biparatopic HER2 antibody zanidatamab, a substance that has not yet been approved, has shown promising data. It binds to two extracellular domains of the HER2 receptor instead of one (8). Like trastuzumab, zanidatamab inhibits HER2-dependent signaling. However, another important effect is a strong immune response based on complement-mediated antibody-dependent cytotoxicity. The first combination study in gastric cancer showed a high ORR with zanidatamab plus chemotherapy (9). The substance is currently being evaluated in phase III.
Combination of trastuzumab with immunotherapy
Recent research results have provided strong arguments in favor of combining immunotherapy and HER2-targeted treatment. This is supported, for example, by the finding that HER2-positive gastric carcinomas more frequently have infiltrating immune cells in the stroma than HER2-negative ones (10). In addition, PD-L1 upregulation was observed in vitro as a potential resistance mechanism to HER2 blockade with trastuzumab (11).
The KEYNOTE-811 study therefore tested pembrolizumab plus trastuzumab and chemotherapy in untreated advanced HER2-positive gastric cancer. Compared to placebo plus trastuzumab and chemotherapy, there were advantages in terms of ORR (73% vs. 60%) and progression-free survival (PFS: 10.0 vs. 8.1 months; HR: 0.73) (12). The final analysis and presentation of the OS data are awaited. According to a press release, the OS endpoint is positive (13). Based on these data, pembrolizumab plus trastuzumab and chemotherapy were approved for the first-line treatment of advanced HER2-positive gastric cancer/GEJ with PD-L1 expression. If a PD-L1 CPS ≥ 1 is detected, the combination should be used as the new standard according to the current ESMO guidelines (14). No effect is expected in PD-L1-negative patients, which is why there is no approval here.
Other GI localizations
Carcinomas in the biliary tract and gallbladder can also express HER2. The ESMO guidelines recommend HER2 evaluation in advanced stages and suggest dual second-line therapy with trastuzumab and pertuzumab in cases of HER2 overexpression (15). Further developments are expected soon in this indication, for example, through the use of T-DXd.
Finally, HER2 is also a potential target in colorectal cancer (CRC). One research group evaluated various HER2-targeted agents in HER2-positive pretreated CRC, with trastuzumab plus lapatinib achieving an ORR of 30% (16). Trastuzumab/pertuzumab also produced an ORR of 32% in another study (17). According to an important finding from this study, trastuzumab/pertuzumab is only effective in a KRAS wild type, as the KRAS mutation confers primary resistance to HER2-targeted agents. In the USA, the combination of tucatinib and trastuzumab has been approved in the setting of HER2-positive and otherwise refractory HER2-overexpressing RAS wild-type metastatic colorectal cancer. This is based on the MOUNTAINEER study, which showed an overall response rate of 38.1% with good overall tolerability for the combination of tucatinib/trastuzumab in this indication (18).
According to the DESTINY-CRC01 study, T-DXd is also effective in metastatic and refractory HER2-positive CRC with RAS and BRAF wild-type: an ORR of 45.3% was reported, with some remissions lasting long (19). Here, only patients with clear HER2 positivity (3+ according to immunohistochemistry or 2+ with positive in situ hybridization) experienced relevant benefits. From the third line onwards, the ESMO guidelines recommend HER2 testing and HER2-targeted therapy for RAS and BRAF wild-type patients (20).
References:
- Hudis C et al.: Trastuzumab – mechanism of action and use in clinical practice. N Engl J Med 2007; 357(1): 39-51
- Nakamura Y et al.: Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: an emerging paradigm. Nat Rev Clin Oncol 2021; 18(8): 473-87
- Kim ST et al.: Impact of genomic alterations on lapatinib treatment outcome and cell-free genomic landscape during HER2 therapy in HER2+ gastric cancer patients. Ann Oncol 2021; 29(4): 1037-48
- Haffner I et al.: HER2 expression, test deviations, and their impact on survival in metastatic gastric cancer: Results from the prospective multicenter VARIANZ study. J Clin Oncol 2021; 39(13): 1468-78
- Swain SM et al.: Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)-related interstitial lung disease/pneumonitis-Focus on proactive monitoring, diagnosis, and management. Cancer Treat Rev 2022; 106: 102378
- Shitara K et al.: Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med 2020; 382(25): 2419-30
- Van Cutsem E et al.: Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): primary and updated analyses from a single-arm, phase 2 study. Lancet Oncol 2023; 24: 744-56
- Weisser NE et al.: An anti-HER2 biparatopic antibody that induces unique HER2 clustering and complement-dependent cytotoxicity. Nat Comm 2023; 14(1): 1394
- Elimova E et al.: Zanidatamab + chemotherapy as first-line treatment for HER2-expressing metastatic gastroesophageal adenocarcinoma (mGEA). J Clin Oncol 2023; 41(Suppl_4): Abstr. #347
- Aisa A et al.: Immune checkpoint inhibitors combined with HER-2 targeted therapy in HER-2 positive gastroesophageal cancer. Crit Rev Oncol Hematol 2022; 180: 103864
- Chaganti BKR et al.: Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNγ secretion. Cancer Lett 2018; 430: 47-56
- Janjigian YY et al.: Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet 2023; 402(10418): 2197-208
- www.merck.com/news/merck-announces-phase-3-keynote-811-trial-met-dual-primary-endpoint-of-overall-survival-os-as-first-line-treatment-in-patients-with-her2-positive-advanced-gastric-or-gastroesophageal-junction-gej
- ESMO Gastric Cancer Living Guideline (www.esmo.org/living-guidelines/esmo-gastric-cancer-living-guideline)
- Vogel A et al.: Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2023; 34(2): 127-40
- Sartore-Bianchi A et al.: Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol 2016; 17(6): 738-46
- Meric-Bernstam F et al.: Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol 2019; 20(4): 518-30
- Strickler JH et al.: Tucatinib plus trastuzumab for chemotherapy-refractory, HER2-positive, RAS wild-type unresectable or metastatic colorectal cancer (MOUNTAINEER): a multicentre, open-label, phase 2 study. Lancet Oncol 2023; 24(5): 496-508
- Yoshino T et al.: Final results of DESTINY-CRC01 investigating trastuzumab deruxtecan in patients with HER2-expressing metastatic colorectal cancer. Nat Commun 2023; 14(1): 3332
- Cervantes A et al: Updated treatment recommendation for third-line treatment in advanced colorectal cancer from the ESMO Metastatic Colorectal Cancer Living Guideline. Ann Oncol 2024; 35(2): 241-3








