Alectinib has been approved by the Swissmedic as a monotherapy for adjuvant treatment in adult patients with ALK-positive stage IB (tumor ≥4cm) to IIIA non-small cell lung cancer after complete tumor resection. The recommended dose is 600 mg twice daily. It is recommended that patients are treated until disease recurrence, uncontrollable toxicity or for a period of 2 years.
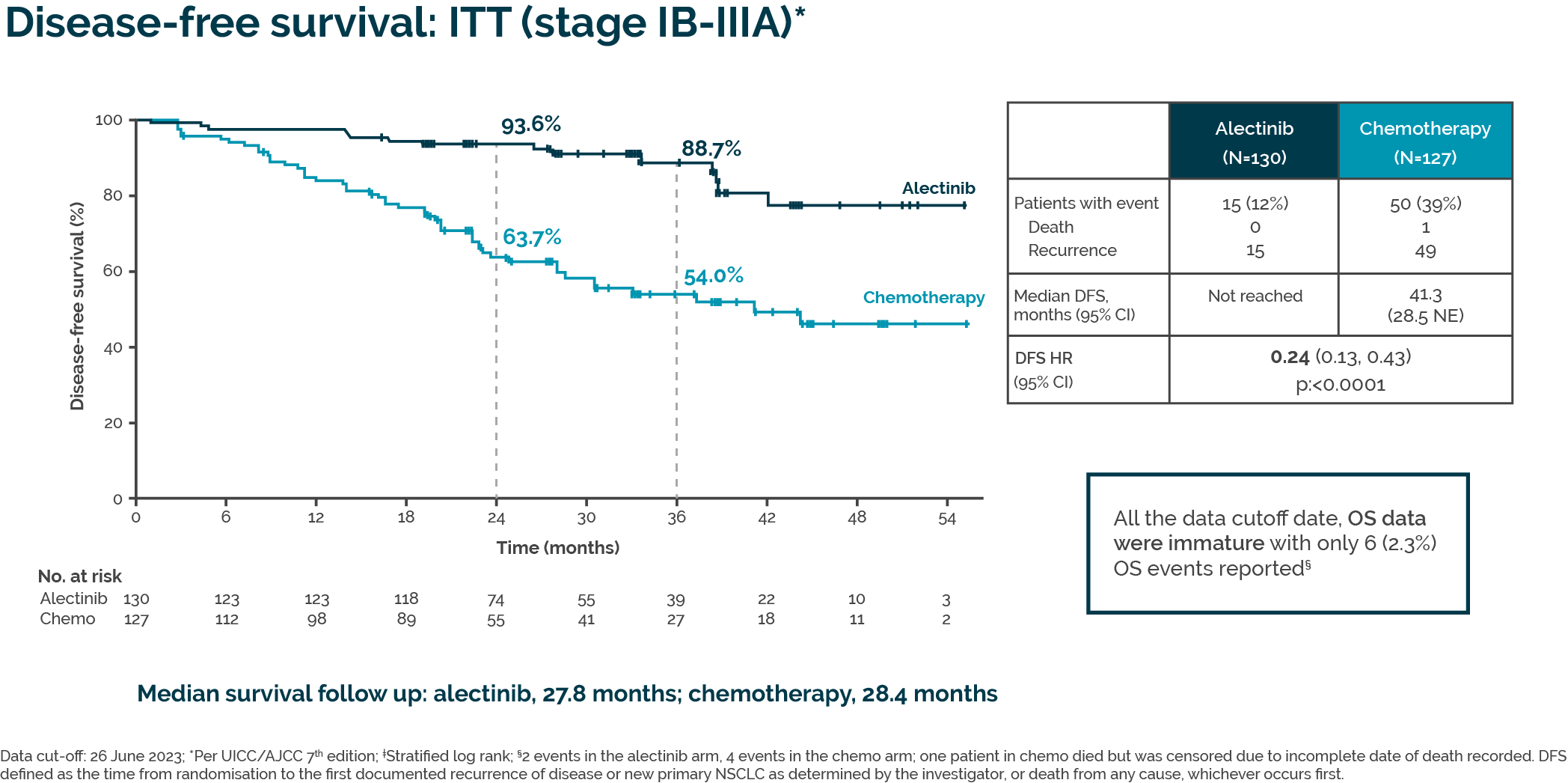
This approval is based on data from the Phase III ALINA trial, which involved 257 patients randomly assigned to receive either alectinib or platinum-based chemotherapy, with disease-free survival as the primary endpoint. Alectinib demonstrated a 76% reduction in the risk of disease recurrence or death compared to platinum-based chemotherapy in patients with completely resected IB (tumor ≥ 4 cm) to IIIA ALK-positive NSCLC.
Additionally, an exploratory analysis revealed an improvement in central nervous system disease-free survival, an important finding given the higher risk of brain metastases in individuals with ALK-positive NSCLC compared to other types of NSCLC.
© OncoViews
Adjuvant alectinib resulted in a statistically significant and clinically meaningful improvement in disease-free survival compared with standard of care chemotherapy in patients with stage IB–IIIA in the ITT population of the ALINA trial. Adapted from Solomon BJ, et al, ESMO Congress 2023, LBA2.
In the study, grade 3–4 adverse events (AEs) were reported in 30% of patients receiving alectinib and 31% receiving chemotherapy, with no grade 5 AEs in either group. Serious AEs occurred in 13% of alectinib patients, compared to 8% on chemotherapy. However, alectinib had fewer serious treatment-related AEs (2% vs. 7%) and treatment withdrawals due to AEs (5% vs. 13%) than chemotherapy, indicating a better tolerability profile.
References:
- swissmedicinfo.ch
- https://www.annalsofoncology.org/article/S0923-7534(23)04195-9/fulltext








