Systemic treatment of ovarian cancer has improved significantly over the last thirteen years thanks to the introduction of targeted substances such as PARP inhibitors. Nevertheless, further research is needed, as recurrences are frequent, and identifying the optimal treatment plan in individual cases is a challenge.
© OncoViews
Great progress has been made in the systemic and surgical treatment of ovarian cancer (OC) in recent decades. Despite this, there is still a significant need for optimized first-line options. According to a 2013 estimate, around 70% of patients recur within three years of first-line therapy (1). Although this figure could change in the near future with the implementation of potent maintenance therapies, a large proportion of patients will continue to suffer recurrences.
For a long time, cytoreductive surgery and chemotherapy formed the pillars of therapeutic management. From the introduction of bevacizumab in 2011, the therapeutic landscape underwent a fundamental change based on the addition of targeted substances to both chemotherapy and maintenance therapy. One important study is the randomized, double-blind phase III PRIMA study, which compared the PARP inhibitor niraparib with placebo as maintenance therapy. Patients with newly diagnosed, high-grade serous or endometrioid advanced ovarian cancer participated after developing a complete or partial response to first-line platinum-based chemotherapy. Around a third were in stage IV, and almost all patients in stage III had a residual tumor after debulking. Homologous recombination deficiency (HRD) was present in half of the 733 randomized patients.
Update on the PRIMA study
According to the primary analysis, progression-free survival (PFS) in the overall population was significantly longer with niraparib than with placebo, regardless of homologous recombination status (13.8 vs. 8.2 months; HR: 0.62; p < 0.001) (2). The update presented at the European Society of Medical Oncology (ESMO) 2024 congress showed a sustained PFS benefit in both the overall group and the HRD cohort (Table 1) (3). No relevant benefit was observed in HR-proficient (HRP) tumors. As expected, the HRD cohort benefited the most, with the PFS rate after five years with niraparib exceeding that with placebo by a factor of two (35 % vs. 16 %). This result is remarkable, as a 5-year PFS rate of 35% in a population with such unfavorable characteristics would have been unthinkable just a few years ago. The PFS curves in the overall group and the HRD cohort remained separate despite the long follow-up period of almost 90 months.
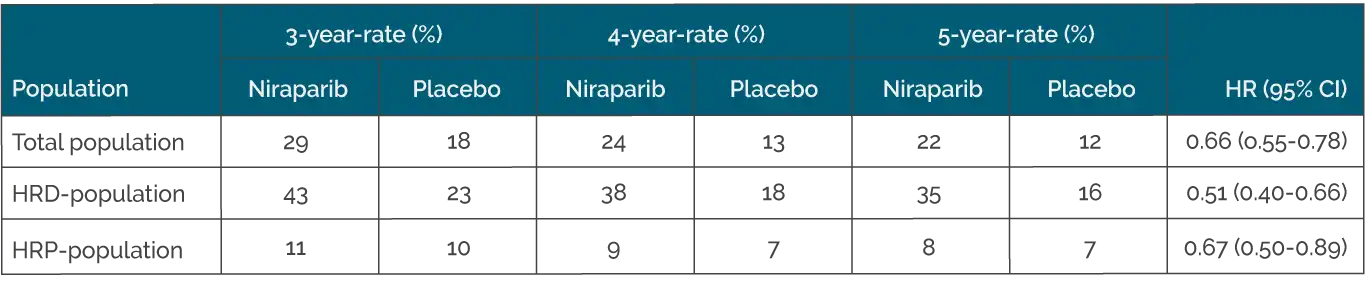
Table 1. Progression-free survival with Niraparib vs. Placebo in the PRIMA study (modified from González-Martín A et al) (3).
However, no significant difference in overall survival was found between the two arms of the PRIMA study, which was true for all populations. The authors point out that the assessment of long-term efficacy in advanced high-risk OC may be complicated by factors such as follow-up therapies. The placebo arm was about three times more likely to receive subsequent PARP inhibition than the niraparib arm; this difference was particularly striking in the HRD/BRCA-mutated cohort. Other possible explanations relate to the very good outcomes in the control arm and the high risk of recurrence in these patients. Overall, the results do not call into question the value of PARP inhibition in the first-line treatment of OC.
PARP inhibitors as a game changer
Even after a follow-up of up to seven years, no new safety signals were observed in the PRIMA study. The rates of myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML) were low at 2.3 % vs. 1.6 % and corresponded to the MDS/AML rates from the Phase III studies on the PARP inhibitors olaparib and rucaparib. Using the globally approved niraparib starting dose, which is adjusted for body weight and platelet count, resulted in a lower incidence of hematologic toxicities than when the fixed-dose was prescribed. According to the author’s conclusion, the long-term results speak in favor of the benefit of first-line maintenance therapy with niraparib, regardless of homologous recombination status.
Given the wealth of data on PARP inhibition, we can tell our patients that this treatment is safe. Maintenance therapy with PARP inhibitors is undoubtedly still a “game changer,” especially for HRD tumors. In the absence of contraindications, the additional administration of bevacizumab should be considered for the group with HRD and residual disease. In the setting of OC with HRP, there is of course a need for other substances. We need to select the ideal systemic therapy and maintenance for each patient depending on factors such as biomarkers, surgical outcomes, response to chemotherapy and toxicity profile, which can be challenging.
Data on Immunotherapy
While immunotherapy has revolutionized the treatment of other gynaecological cancers, such as endometrial cancer, there is still much to learn in OC. One example of dashed expectations is the phase III ATHENA-COMBO trial, which tested maintenance therapy with the combination of rucaparib and nivolumab in newly diagnosed stage III-IV OC (4). The control arm received rucaparib monotherapy. The patients had achieved a complete or partial response after the “frontline” administration of a platinum doublet and surgery. Although rucaparib performed as expected, the combination with nivolumab was not only superior to rucaparib monotherapy but actually inferior (median PFS: 15.0 vs. 20.2 months; HR: 1.29).
Overall, it can be concluded that the addition of PD-(L)1 inhibitors to chemotherapy should be avoided in high-grade serous OC, as further research is still required. This does not mean that immunotherapy should not be further evaluated, but obviously, the situation is more complicated in OC than in other types of cancer.
There are currently almost 190 antibody-drug conjugates in development worldwide, and the number of targets is growing continuously. Here we have opened Pandora’s box, and OC is no exception. However, it will be necessary to figure out how these new therapies should fit into the OC treatment landscape.
Conclusion
In recent years, we have learned a lot and made numerous advances, especially for patients with BRCA-mutated/HRD tumors. While the systemic therapy landscape has changed rapidly, the current challenge is to determine which tools should be used first in each individual case and which should be saved for later. This brings with it the need to better characterize patient groups, identify robust biomarkers and define specific treatment algorithms.
At present, HRD status is the only valid biomarker, although ultimately the response to platinum therapy and surgical quality are the decisive factors. Of course, the burden of surgical and systemic therapies and their toxicities must be weighed against the actual treatment benefit.
Explore more about the latest gynecological cancers with Drs. Apostolos Sarivalasis, Ilaria Colombo, and Christina Fotopoulou.
References
- J. A. Ledermann et al., “Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up,” Annals of Oncology, vol. 24, pp. vi24–vi32, Sep. 2013, doi: 10.1093/annonc/mdt333.
- A. González-Martín et al., “Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer,” New England Journal of Medicine, vol. 381, no. 25, pp. 2391–2402, Sep. 2019, doi: 10.1056/nejmoa1910962.
- A. González-Martín et al., “LBA29 Final overall survival (OS) in patients (pts) with newly diagnosed advanced ovarian cancer (aOC) treated with niraparib (nir) first-line (1L) maintenance: Results from PRIMA/ENGOT-OV26/GOG-3012,” Annals of Oncology, vol. 35, pp. S1222–S1223, Sep. 2024, doi: 10.1016/j.annonc.2024.08.2268.
- Monk BJ et al., “ATHENA-COMBO, a phase III, randomized trial comparing rucaparib (RUCA) + nivolumab combination therapy vs. RUCA monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer.” Annals of Oncology (2024) 35 (suppl_2): 1-72. 10.1016/annonc/annonc1623








