Dr. Terence Friedlander, a medical oncologist and professor of medicine at the University of California, San Francisco, recently discussed key insights from clinical practice regarding the combination of enfortumab vedotin (EV) and pembrolizumab in patients with treatment-naïve metastatic urothelial cancer. His discussion provided a comprehensive overview of the rationale behind combining these agents, the clinical trial data supporting their use, and the management of associated toxicities.
Enfortumab vedotin (EV) is a nectin-4-directed antibody-drug conjugate that delivers an MMAE (monomethyl auristatin E) payload. Initially evaluated as a monotherapy in late-line trials for advanced bladder cancer treatment, it was subsequently combined with pembrolizumab, and investigated further by the phase III EV-302 trial.
The drug functions by binding to nectin-4 on tumor cells, leading to the internalization and release of MMAE, a synthetic antineoplastic agent, which disrupts microtubules and induces apoptosis. This process generates immunostimulatory signals, recruiting T cells and antigen-presenting cells, which provide a biological rationale for combining EV with pembrolizumab, a PD-1 inhibitor, to further enhance tumor cell killing (1).
The EV-302 Trial: Key Findings
The EV-302 phase III trial compared EV plus pembrolizumab to chemotherapy (cisplatin or carboplatin-based regimens with gemcitabine) in nearly 900 patients, randomized in a 1:1 ratio (2). The primary endpoints were overall survival (OS) and progression-free survival (PFS). Notably, there was no maximum number of EV cycles in the trial, whereas chemotherapy was limited to six cycles. As a result, patients received different amounts of EV, which resulted in different levels of toxicity.
Findings from the trial showed a median progression-free survival (PFS) of 12.5 months in the EV-P arm, nearly double that of the chemotherapy arm, which had a median PFS of 6.3 months (hazard ratio <0.45). Additionally, the median overall survival (OS) was significantly longer in the EV-P arm, reaching 31.5 months compared to 16.1 months in the chemotherapy arm (hazard ratio: 0.47) (Figure 1). These results established EV and pembrolizumab as a standard frontline option for patients with locally advanced and metastatic urothelial cancer (la/mUC).

Figure 1. Overall survival (OS) analysis of enfortumab vedotin (EV) vs chemotherapy in previously untreated locally advanced / metastatic urothelial cancer. Adapted from Powels et al. (2)
Despite the improvements in OS and PFS, the trial highlighted treatment-emergent adverse events (AEs) associated with the EV and pembrolizumab combination, some occurring as early as cycle one (Fig. 2). In the EV-P arm, peripheral sensory neuropathy and skin-related issues—such as pruritus, alopecia, and rash—were more prevalent. In contrast, AEs like nausea and myelosuppression were significantly more common in the chemotherapy group. According to Dr. Friedlander, it is essential to carefully assess these toxicities and implement appropriate management strategies when considering the use of EV and pembrolizumab.
In his recent talk, Dr. Friedlander highlighted five major side effects: dermatologic toxicity, peripheral neuropathy, pneumonitis, ocular toxicity, and hyperglycemia. To discuss potential management strategies, he presented case studies as examples.
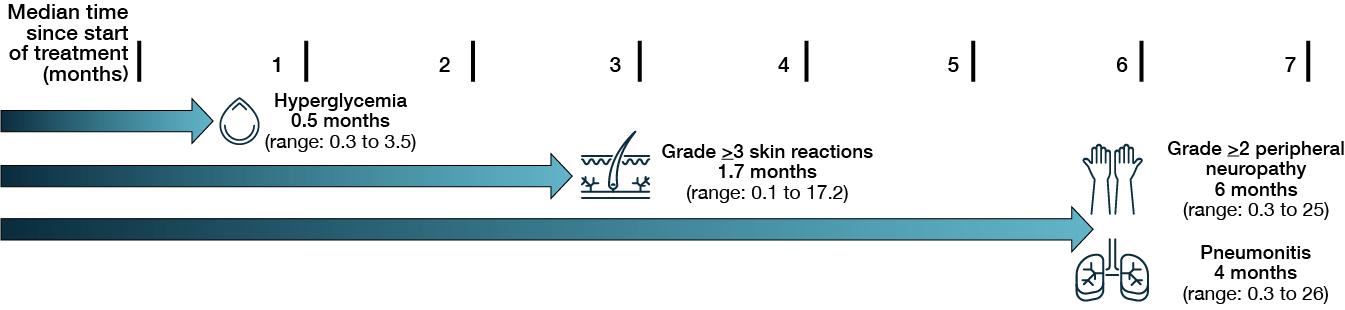
Figure 2. Median times to onset of select adverse events in patients with locally advanced metastatic urothelial cancer treated with enfortumab vedotin plus pembrolizumab. Taken from Brower et al. (3).
Managing Dermatologic Toxicity
A 72-year-old woman with a history of non-muscle-invasive bladder cancer and prior BCG therapy developed metastatic urothelial cancer and started EV-pembrolizumab as frontline therapy. Within days, she developed a grade 2 diffuse, pruritic, red rash covering her trunk, back, and arms. Nectin-4 is highly expressed in the skin, making dermatologic toxicity an on-target off-tumor effect and a classic AE of EV. (4)
Grade 1-2 skin toxicity has been observed in 50-70% of patients, with 10-20% experiencing severe (grade 3) reactions across trials. It commonly presents as a maculopapular rash, alopecia, hyperpigmentation, or—in severe cases—blistering, raising concern for Stevens-Johnson syndrome or toxic epidermal necrolysis (4-6).
Recognizing and managing skin toxicities requires a multidisciplinary approach involving healthcare providers, patients, and caregivers. However, differentiating between EV-related and pembrolizumab-related skin reactions can be challenging. EV-related skin toxicities typically develop early, within the first 2–3 treatment cycles, and are more common in skin folds such as the fingers, axilla, and groin. In contrast, pembrolizumab-related skin reactions tend to appear later in treatment. In complex cases, dermatology consultation may be necessary to determine the underlying cause and guide appropriate management.
Dr. Friedlander goes on to outline his management approach based on the severity of skin toxicity. For grade 1 toxicity, high-potency topical steroids are recommended, and EV therapy can continue without dose reduction. In cases of grade 2 toxicity, systemic steroids such as oral prednisone should be used along with emollients, and a skin biopsy may be considered. EV should be temporarily discontinued, with a dose reduction when resuming treatment. Grade 3 toxicity, characterized by rashes covering more than 10% of the body surface area along with symptoms such as fevers, blistering, or unexplained liver or kidney changes, requires hospitalization, dermatology consultation, IV corticosteroids, and discontinuation of EV. (4-5)
In this patient’s case, the EV was held and oral prednisone was initiated, leading to rapid improvement. She resumed therapy at a reduced dose, managing further skin toxicities with topical steroids and antihistamines. By cycle three, her tumor showed signs of response, and her skin toxicities were well controlled.
Managing Neuropathy
During cycle four, the same patient developed difficulty with balance and fine motor tasks, such as tying her shoes, suggesting new-onset neuropathy. Neuropathy, another common AE of EV, affects nearly 50% of patients, with 25% experiencing moderate to severe symptoms (7-8).
Early intervention is critical (Fig 3). Dr. Friedlander’s suggested management includes dose reduction at grade 1, including treatment breaks to help prevent progression to grade 2 neuropathy, which can significantly impact quality of life. In this case, the patient developed grade 2 sensory neuropathy and grade 1 motor neuropathy. In this patient’s case, EV was held for three weeks, leading to slight improvement and she resumed treatment at a lower dose. By cycle six, the patient achieved a complete response but remained on pembrolizumab alone due to persistent neuropathy.

Figure 3. Risk factors, prevention strategies, monitoring, and management recommendations for treatment-emergent peripheral neuropathy. Taken from Brower et al. (3)
Managing Pneumonitis
Dr. Friedlander also presented a 73-year-old woman who was admitted with urinary retention and diagnosed with high-grade urothelial carcinoma, including a plasmacytoid variant, an aggressive subtype. She initially received platinum-based chemotherapy but had advanced T4 N2 disease at the time of surgery. Following surgery, she underwent six months of adjuvant nivolumab; however, a PET-CT later revealed new retroperitoneal lymph nodes and bone metastases. In mid-2024, she began treatment with EV at full dose alongside pembrolizumab, despite uncertainty about pembrolizumab’s efficacy following prior nivolumab use. She tolerated the first few cycles well, but within two months, she developed a cough, worsening fatigue, dyspnea on exertion, and hypoxemia. A follow-up chest CT revealed clear infiltrates in her left lung, raising concerns about treatment-related pneumonitis.
Pneumonitis is a known side effect of EV (Fig 4), occurring in about 10% of patients in the EV-302 trial (2). This toxicity is likely related to MMAE, the cytotoxic component of EV, as similar effects have been observed with other MMAE-containing antibody-drug conjugates. Since nectin-4, EV’s target, is rarely expressed in healthy lung tissue, this is likely an off-target effect. Distinguishing between EV-related and pembrolizumab-related pneumonitis is challenging since both drugs can cause it (6,9).
A Korean study of 64 patients found pneumonitis in 18 individuals, with a 3% mortality rate, most commonly presenting as organizing pneumonia (10). The median onset was around 13 weeks. Notably, pneumonitis is less common with pembrolizumab, occurring in only 4% of patients, making EV the more likely culprit in this case. For this reason, the patient management involved holding both drugs and initiating a prednisone taper, leading to rapid improvement. The patient recovered so well that bronchoscopy was deferred, and a follow-up CT scan five weeks later showed significant resolution of lung lesions.
Given her high-risk disease and a strong desire to continue treatment, EV was cautiously reintroduced at a low dose (0.56 mg/kg). However, a week later, she developed new dyspnea and hypoxemia, indicating recurrent pneumonitis. After another round of prednisone, her condition improved, but EV was permanently discontinued. Unfortunately, her disease progressed and alternative late-line treatment options are being considered.
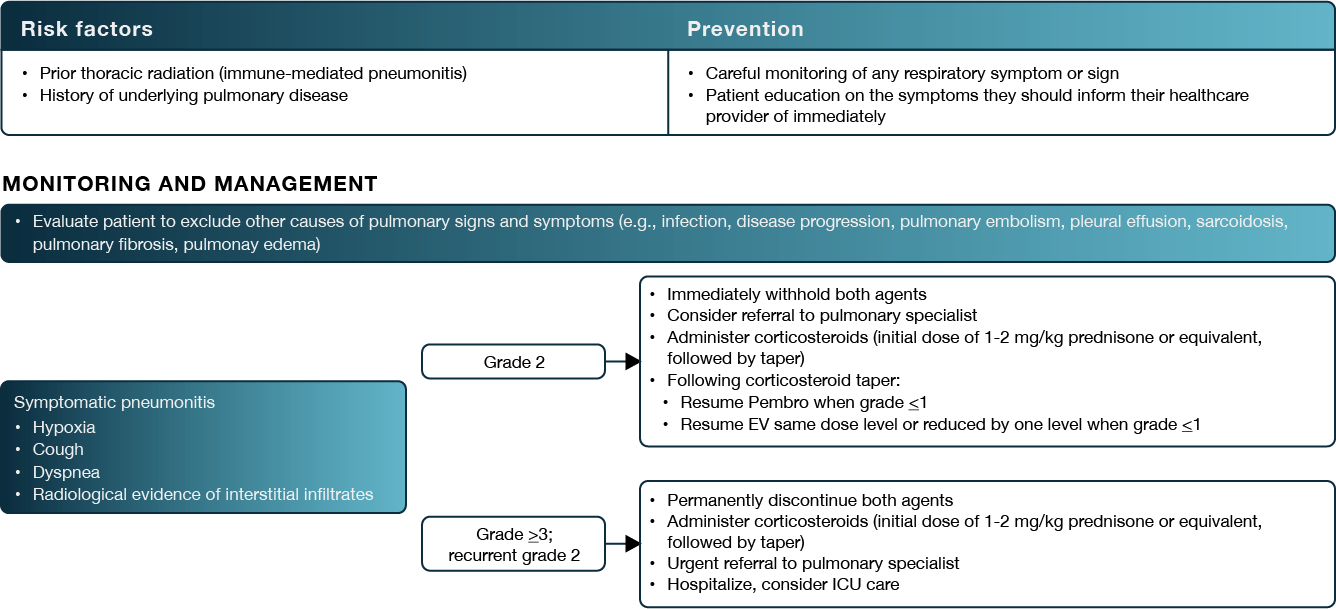
Figure 3. Risk factors, prevention strategies, monitoring, and management recommendations for treatment-emergent pneumonitis. Taken from Brower et al. (3)
Managing Other Major Toxicities
In addition to pneumonitis, other important toxicities associated with EV include ocular toxicity and hyperglycemia (4). Ocular toxicity occurs due to nectin-4 expression in the eye, often presenting as a keratitis-like condition. While not extremely common—affecting about 20% of patients—it can be managed with lubricating eye drops and, in some cases, ophthalmologist-prescribed steroids. Referral to an ophthalmologist is recommended when symptoms arise.
Hyperglycemia is also a known class effect of MMAE, with early studies of MMAE-containing antibody-drug conjugates reporting cases of diabetic ketoacidosis, some fatal. Due to this risk, EV should not be used in patients with an HbA1c greater than 8% or blood glucose levels exceeding 250 mg/dL. Blood sugar levels should be checked before each dose, and patients with borderline or uncontrolled diabetes should be referred to endocrinology for proper management before initiating EV.
While skin toxicity and neuropathy are common side effects of EV-pembrolizumab, they can be effectively managed through proactive dose adjustments. Pneumonitis, though less frequent, requires early detection and a multidisciplinary approach, including pulmonology referral, to ensure proper management. Ocular toxicity and hyperglycemia should also be closely monitored to prevent complications. Implementing careful adverse event recognition and management strategies helps patients remain on therapy longer, increasing the likelihood of durable responses. Dr. Friedlander emphasized that by tailoring treatment to each patient’s needs and addressing side effects proactively, clinicians can maximize the therapeutic benefits of EV-pembrolizumab while prioritizing patient safety and quality of life.
Watch the full video to explore more on managing adverse events of enfortumab vedotin and pembrolizumab with Dr. Terence Friedlander.
References
- Shilpa Gupta et al., Study EV-103 dose escalation/cohort A: Long-term outcome of enfortumab vedotin + pembrolizumab in first-line (1L) cisplatin-ineligible locally advanced or metastatic urothelial carcinoma (la/mUC) with nearly 4 years of follow-up. JCO 41, 4505-4505(2023).
- Thomas B. Powles et al., LBA6 EV-302/KEYNOTE-A39: Open-label, randomized phase III study of enfortumab vedotin in combination with pembrolizumab (EV+P) vs chemotherapy (Chemo) in previously untreated locally advanced metastatic urothelial carcinoma (la/mUC). Ann. Oncol 34, S1340 (2023).
- Blaine Brower et al., Managing potential adverse events during treatment with enfortumab vedotin + pembrolizumab in patients with advanced urothelial cancer. Front. Oncol 14: 1326715 (2024).
- Mario E Lacouture et al., Management of dermatologic events associated with the Nectin-4-directed Antibody-Drug conjugate enfortumab vedotin. The Oncologist 27(3), e223-e232 (2022).
- Allison S Dobry et al., Cutaneous reactions with enfortumab vedotin: A case series and review of the literature. JAAD Case Reports 14, 7-9 (2021).
- Sam Wu et al., Cutaneous toxicity associated with enfortumab vedotin treatment of metastatic urothelial carcinoma. Dermatology Online Journal 25, 2 (2019).
- Rebecca L. Best et al., Microtubule and tubulin binding and regulation of microtubule dynamics by the antibody drug conjugate (ADC) payload, monomethyl auristatin E (MMAE): Mechanistic insights into MMAE ADC peripheral neuropathy. Toxicol. Appl. Pharmacol 421, 115534 (2021).
- Thomas Powles et al., Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med 384, 1125-1135 (2021).
- Joaquim Bellmunt, et al. Pembrolizumab as Second-Line therapy for Advanced Urothelial Carcinoma. N Engl J Med 376, (11):1015-1026 (2017).
- Shinkyo Yoon, et al., Enfortumab vedotin-related pneumonitis is more common than expected and could lead to acute respiratory failure. Eur. J. Cancer 174, 81-89 (2022).