Detecting biomarkers is a key tool in the treatment of lung cancer. Testing is now also carried out in the early stages, enabling a growing variety of treatment options for metastatic disease. Challenges in everyday clinical practice and unanswered questions underline the need for continuous optimization.
Non-small cell lung cancer (NSCLC) is a prime example of a disease that is diagnosed and treated based on the principles of precision medicine. In view of the high recurrence rates, even in the early stages of the disease, precision medicine has also found its way into these areas. Biomarkers are therefore tested both in operable lung cancer and in stages III and IV.
Neoadjuvant and adjuvant setting
Based on the Checkmate-816 study, neoadjuvant nivolumab was approved in combination with chemotherapy in resectable, PD-L1-expressing NSCLC with a high risk of recurrence (1,2). The study evaluated various biomarkers, including tumor mutation burden, PD-L1 expression, and circulating tumor DNA (ctDNA). It was found that only PD-L1 expression ≥ 1% was predictive of survival. There was a correlation between ctDNA clearance and the rate of pathological complete remissions (Figure 1), which is why research is currently in full swing to further explore the significance of ctDNA analysis. In the field of targeted therapies, there are still no approvals in the neoadjuvant setting. Osimertinib is being investigated in NeoADAURA for EGFR-positive resectable tumors (NCT04351555), and further clinical trials are currently underway.
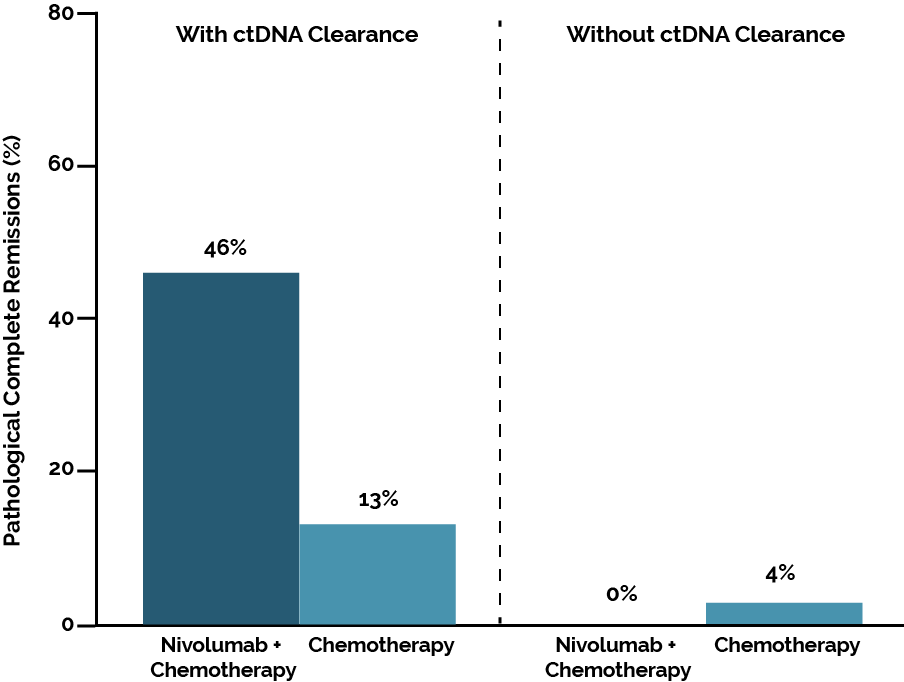
Figure 1. Pathological complete remissions depending on ctDNA clearance in CheckMate 816. Adapted from Forde PM et al (2).
In the adjuvant setting, immunotherapy with atezolizumab or pembrolizumab was established as the standard of care based on the IMpower010 and PEARLS/KEYNOTE-091 trials (3,4). However, both trials lacked uniform guidelines about biomarker testing (PD-L1, ALK, EGFR). In EGFR-mutated NSCLC, the ADAURA study was able to demonstrate clinical benefits from adjuvant osimertinib (5). As a result, it is essential to determine the EGFR mutation status in early NSCLC. This also applies to the testing of ALK rearrangements, as the data from the ALINA study demonstrate the benefit of adjuvant alectinib (6). To combine the advantages of neoadjuvant and adjuvant therapy, the perioperative treatment strategy is currently being clinically tested. However, biomarker testing is not yet well established in this setting.
Assistance from AI
The accurate evaluation of PD-L1 expression poses a particular challenge for pathologists. Scores and cut-offs must be taken into account, and the enormous heterogeneity of this parameter must be considered. Artificial intelligence will certainly help to improve processes in the near future. Evaluating tumor regression as a result of neoadjuvant therapy is also not an easy task, but established criteria defined by the International Association for the Study of Lung Cancer can help (7). However, it must be ensured that all pathologists apply these criteria when evaluating samples after neoadjuvant treatment.
Overall, it can be stated that biomarker testing has advanced the field of early lung cancer. The panel should at least include PD-L1 expression as well as ALK and EGFR status. Whether comprehensive testing is already recommended at the time of diagnosis of early lung cancer is up for discussion. In the extremely heterogeneous stage III, there is little evidence for biomarker-based treatment, which is why decisions should be made on an interdisciplinary basis. The LAURA study on the use of osimertinib after chemoradiotherapy of resectable, EGFR-mutated tumors provides an example of successful biomarker-based therapy (8). When monitoring the response to consolidating immunotherapy in stage III, ctDNA analysis could become a valuable tool (9).
Stumbling blocks in stage IV
A constantly growing group of targeted therapies is available for a large group of stage IV patients. Pathologists are confronted with the difficulty of having to examine small tissue biopsies with multiple assays under time pressure. Panel-based methods that include fast-track strategies, in which the most important markers for first-line therapy are recorded within 1-3 days, prove to be time- and tissue-saving (10).
Special attention has only recently been paid to EGFR exon 20 insertions and HER2 mutations, as targeted drugs are now available. In the field of checkpoint inhibitors, PD-L1 expression, and tumor mutation burden are not known to be perfect markers in terms of prediction. Combinations of STK11, KEAP1, and KRAS mutations have been associated with a reduced response to immunotherapy (11). The biggest challenge in the context of precision medicine results from the fact that a substantial proportion of patients with NSCLC do not receive any testing or, even if a test result is available, do not always receive an appropriate targeted therapy (12). In Germany, a broad network has been implemented that allows all patients with NSCLC to be tested. Unfortunately, the need for testing is insufficiently known, especially in the early stages, which is reflected in the continued low testing rates (13).
Conclusion
In summary, the importance of biomarker testing as an indispensable tool in lung cancer must be emphasized. We need to design “smart” panels that allow us to get results with little tissue in a short time frame. In my opinion, ctDNA will establish itself as a valuable biomarker, especially in the context of monitoring, and AI-supported tools will facilitate the standardized evaluation of markers such as PD-L1 expression. I see the most important task as the continuous training of our colleagues about the necessity of biomarker testing in NSCLC patients, even in the early stages.
This article is based on the talk by Dr. Anne Maria Schultheis at the International Lung Cancer Summit 2024.

References
- Fachinformation Opdivo®
- Forde PM et al: Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med 2022; 386(21): 1973-19853
- Felip E et al: Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021; 398(10308): 1344-1357
- O’Brien M et al: Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol 2022; 23(10): 1274-1286
- Wu YL et al: Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med 2020; 383(18): 1711-1723
- Wu YL et al: Alectinib in resected ALK-positive non-small-cell lung cancer. N Engl J Med 2024; 390(14): 1265-1276
- Travis WD et al: IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J Thorac Oncol 2020; 15(5): 709-740
- Lu S et al: Osimertinib after chemoradiotherapy in stage III EGFR-mutated NSCLC. N Engl J Med 2024; 391(7): 585-597
- Jun S et al: Analysis of circulating tumor DNA predicts outcomes of short-course consolidation immunotherapy in unresectable stage III NSCLC. J Thorac Oncol 2024; 19(10): 1427-1437
- Schultheis RM et al: Precision medicine in non-small cell lung cancer: Current applications and future directions. Seminars in Cancer Biology 2020; 84:184-198
- Ricciuti B et al: Diminished efficacy of programmed death-(ligand)1 inhibition in STK11- and KEAP1-mutant lung adenocarcinoma is affected by KRAS mutation status. J Thorac Oncol 2022; 17(3): 399-410
- Sadik K et al: Impact of clinical practice gaps on the implementation of personalized medicine in advanced non-small-cell lung cancer. JCO Precis Oncol 2022; 6: e2200246
- Loges S et al: Aktuelle Umfrage zur Testbereitschaft in frühen und späten Stadien des NSCLC. Journal Onkologie 5/2024. https://www.journalonko.de/artikel/lesen/umfrage-testbereitschaft-nsclc-deutschland