Dr. Jens Huober, head of the Breast Center at Cantonal Hospital St. Gallen, presented an in-depth analysis of recent advancements in the treatment of early-stage triple-negative breast cancer (TNBC) from this year’s ESMO conference. Focusing on key findings from the updated KEYNOTE-522 trial, Prof. Huober outlined promising results that impact clinical practice for high-risk TNBC patients.
© OncoViews
KEYNOTE-522 Trial: Design and Treatment Overview
The KEYNOTE-522 trial, a major study in the field of neoadjuvant TNBC treatment, enrolled patients with high-risk, non-metastatic TNBC. Eligible participants had tumors larger than two centimeters or node-positive disease, and all patients received an initial regimen of chemotherapy with carboplatin and paclitaxel, followed by epirubicin and cyclophosphamide. The patients were then randomized to either pembrolizumab (200 mg every three weeks) or placebo. Following chemotherapy and surgery, those in both groups continued their assigned treatment for nine additional cycles. The trial’s primary endpoints were pathologic complete response (pCR) rate and event-free survival (EFS), and the study was designed to evaluate whether pembrolizumab could offer a significant survival benefit over standard chemotherapy alone.
Primary Endpoint Data: Pathologic Complete Response and Event-Free Survival
Dr. Huober highlighted the primary endpoint data, which showed a significantly higher pCR rate for patients receiving pembrolizumab: 65%, compared to 51% in the placebo group. This improvement in pCR was further linked to a better EFS in pembrolizumab-treated patients, with EFS rates of 81.3% at five years, compared to 72.3% in the placebo group. These findings suggest that pembrolizumab may play a significant role in improving long-term outcomes for TNBC patients, a group traditionally associated with poor prognosis and limited treatment options.
Overall Survival Data: Survival Benefit Across Subgroups
New data presented at ESMO 2024 revealed an overall survival (OS) advantage for pembrolizumab. This benefit was consistent across multiple subgroups, including patients with both positive and negative nodal status, different tumor sizes, and varying PD-L1 statuses, yielding a hazard ratio of 0.66 and a statistically significant p-value of 0.015. Importantly, both PD-L1 positive and PD-L1 negative patients benefited from pembrolizumab treatment, reinforcing the potential utility of this therapy across a broad spectrum of TNBC patients.
pCR and Prognosis: Pembrolizumab’s Role in Residual Disease
Dr. Huober noted that patients achieving pCR had superior survival outcomes compared to those without pCR. However, he emphasized that pembrolizumab still provided a survival advantage even for those with residual disease. Dr. Huober also noted that the survival benefit in pCR patients was small—approximately 1%—prompting questions about the necessity of adjuvant pembrolizumab for those who achieve pCR. For patients without pCR, pembrolizumab still offered benefits, but these patients generally had poorer outcomes, suggesting a need to explore additional treatment options, such as antibody-drug conjugates (ADCs), for those with residual disease.
Pembrolizumab did not introduce any unexpected side effects, but immune-mediated adverse events were higher compared to the placebo group. The most common side effects included hypothyroidism and severe skin reactions, reflecting the typical safety profile seen with immune checkpoint inhibitors.
Considerations for Stage I TNBC
One of the ongoing challenges in TNBC management, Dr. Huober explained, involves optimizing treatment for stage I disease. The KEYNOTE-522 trial provided clear guidelines for stage II and III patients, but the role of neoadjuvant versus adjuvant therapy for stage I patients remains unclear. He discussed a recent study from the Netherlands, presented at ESMO, which included 1,144 patients with stage I TNBC who received neoadjuvant therapy. This study showed an impressive four-year OS rate of 98% for patients achieving pCR, with a 93% survival rate for those with residual disease. Additionally, about half of these patients received platinum-based chemotherapy, with adjuvant capecitabine administered to a minority (approximately 25%).
Another Dutch study suggested that TILs might be useful for guiding TNBC treatment, especially in stage I patients. High TIL levels (≥75%) were associated with excellent outcomes even in the absence of chemotherapy. Dr. Huober noted that this biomarker could one day help clinicians identify TNBC patients who may safely avoid chemotherapy, depending on their TIL count.
Institutional Treatment Algorithm for TNBC
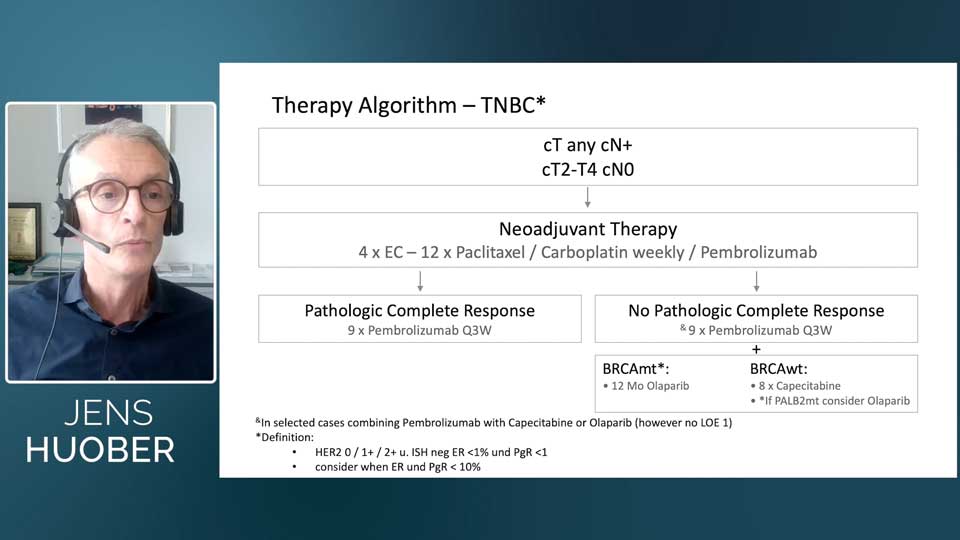
Based on these insights, Dr. Huober shared a treatment algorithm his institution uses for TNBC patients. For tumors larger than 2 cm or node-positive disease, they follow the KEYNOTE-522 protocol: carboplatin-paclitaxel, followed by epirubicin-cyclophosphamide, in combination with pembrolizumab. All patients continue pembrolizumab for nine additional cycles, regardless of pCR status. In high-risk patients with BRCA mutations, olaparib may be added to pembrolizumab; in BRCA wild-type patients, capecitabine is considered alongside pembrolizumab.
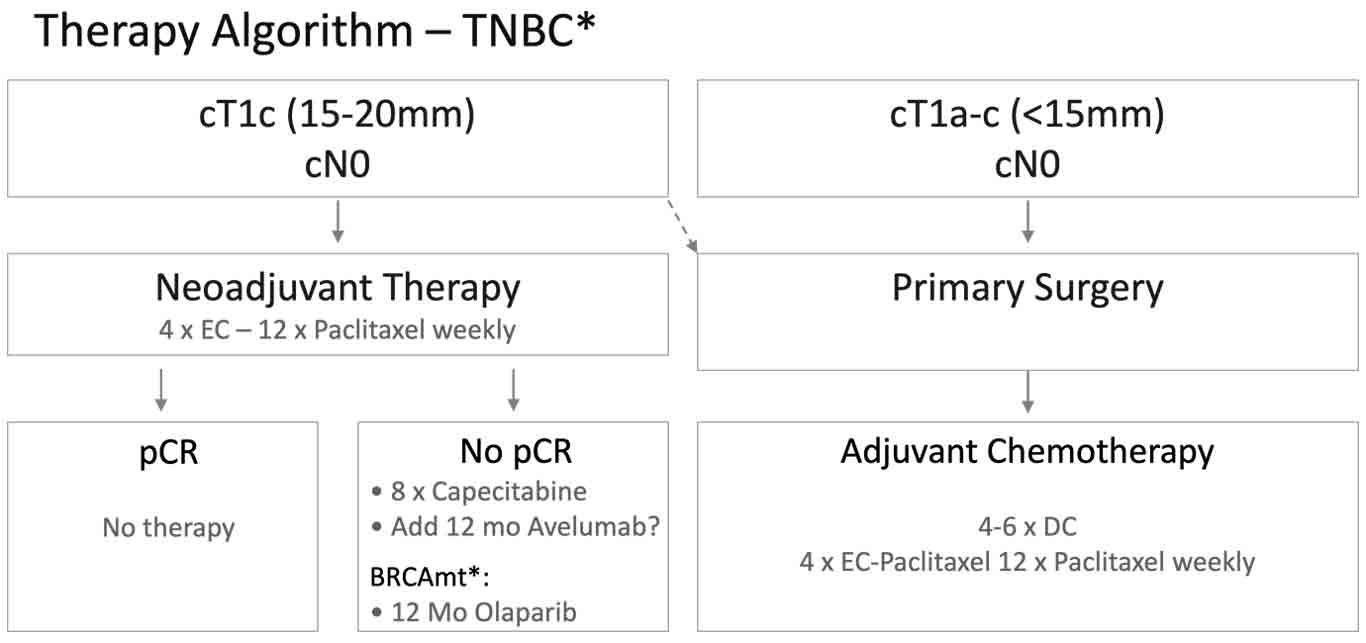
© Jens Huober, MD
Fig. 1. Treatment algorithm for patients with triple-negative breast cancer, designed by the Breast Center at Cantonal Hospital St. Gallen.
For smaller stage I tumors, the approach varies based on tumor size. Tumors under 1.5 cm are treated with surgery first, followed by adjuvant docetaxel-cyclophosphamide or a regimen of EC and weekly paclitaxel. Tumors nearing 2 cm are more likely to receive neoadjuvant anthracycline-taxane therapy. Patients without pCR receive adjuvant capecitabine, and those with BRCA mutations may receive a year of olaparib.
Redefining “Triple-Negative”: A Grey Area in Classification
As a final point, Dr. Huober noted that the TNBC classification is evolving. KEYNOTE-522 defined TNBC as tumors with ER and PgR expression under 1%, yet some emerging data suggest expanding this definition to include ER and PgR expression under 10%. He emphasized that this “grey zone” could have implications for treatment, though further research is needed to clarify its impact.
Conclusion
Dr. Huober’s presentation underscored significant advances in the treatment of TNBC, especially through the use of pembrolizumab in high-risk cases. His discussion also highlighted unresolved questions, such as the management of stage I disease and the role of novel biomarkers like TILs. With evolving definitions and an increasing understanding of individual risk factors, Dr. Huober’s insights indicate that TNBC treatment is poised to become more personalized, offering new hope to patients in this challenging subtype of breast cancer.
Watch the full expert talk with Dr. Jens Huober to explore more on the ESMO 2024 Highlights in TNBC.
References
P. Schmid et al., “LBA4 Neoadjuvant pembrolizumab or placebo plus chemotherapy followed by adjuvant pembrolizumab or placebo for high-risk early-stage TNBC: Overall survival results from the phase III KEYNOTE-522 study,” Annals of Oncology, vol. 35, pp. S1204–S1205, Sep. 2024, doi: 10.1016/j.annonc.2024.08.2247.
M. De Graaf, R. Gielen, S. Balduzzi, S. Siesling, S. Linn, and M. Kok, “236MO Pathologic complete response and survival after neoadjuvant chemotherapy in stage I TNBC: A registry-based study,” Annals of Oncology, vol. 35, p. S311, Sep. 2024, doi: 10.1016/j.annonc.2024.08.179.
P. Schmid et al., “Overall Survival with Pembrolizumab in Early-Stage Triple-Negative Breast Cancer,” New England Journal of Medicine, Sep. 2024, doi: 10.1056/nejmoa2409932.
P. Schmid et al., “Abstract LBO1-01: Neoadjuvant pembrolizumab or placebo plus chemotherapy followed by adjuvant pembrolizumab or placebo for early-stage triple-negative breast cancer: Updated event-free survival results from the phase 3 KEYNOTE-522 study,” Cancer Research, vol. 84, no. 9_Supplement, pp. LBO1-01, May 2024, doi: 10.1158/1538-7445.sabcs23-lbo1-01.