As with other types of cancer, the therapeutic landscape for cervical cancer has been transformed by immunotherapy, but changes are taking place in various strategic areas. Innovative approaches are expanding the algorithms for locally advanced and metastatic disease.
Estimates from 2021 list cervical cancer (CC) as the second most common gynecologic cancer and fourth most common cause of cancer-related death in women worldwide (1). Human papillomavirus (HPV) infections are considered the main risk factor; more than 99% of cases are due to persistent high-risk genital infection (2). Globally, (locally) advanced CC is more common in low- to middle-income countries due to unequal access to screening and treatment (3).
Squamous cell carcinoma is the most common CC subtype (4). There are also adenocarcinomas and some rare histologies, such as those that are not associated with HPV and therefore cannot be prevented by vaccination. The interpretation of the most recent studies on locally advanced CC (LACC) is complicated by the fact that staging has evolved since the 2009 and 2014 FIGO classifications used in the INTERLACE and KEYNOTE-A18 studies to the ninth version of AJCC-TNM staging (FIGO 2021) used today (5-7).
Induction Chemotherapy Plus CRT: INTERLACE
Radiation has always been the central pillar of treatment for LACC. The addition of concomitant chemotherapy further extended overall survival (OS), making cisplatin-based chemoradiotherapy (CRT) the standard of care (8). Among the strategies tested for outcome optimization, induction chemotherapy (ICT) administered prior to CRT showed efficacy. The randomized phase III INTERLACE trial evaluated ICT in the form of carboplatin AUC2 for six weeks plus paclitaxel 80 mg/m2/week followed by standard CRT in patients with newly diagnosed CC in stages 1B1 (node-positive), IB2, II, IIIB or IVA according to FIGO 2009 with different histologies. The control group received CRT only. Both arms included 250 people each, most of whom were in stage II. The co-primary endpoints were progression-free survival (PFS) and OS.
According to the data presented at the ESMO Congress 2023, the addition of ICT led to a PFS extension with a risk reduction of 35 % (HR: 0.65; p = 0.013) (9). After five years, 73 % vs. 64 % of patients were progression-free. Similarly favorable results were achieved for OS, with mortality reduced by almost 40 % (HR: 0.61; p = 0.04). These results have fundamentally changed the treatment perspective for LACC. Data from the INTERLACE study presented at the ESMO Congress 2024 demonstrated no clinically relevant reduction in quality of life as a result of ICT (10).
Immuno-Oncology Therapies Plus CRT Followed By Maintenance: KEYNOTE-A18
The rationale for evaluating immunotherapy in CC was based on the high concentration of CD8+ lymphocytes and PD-1 in the tumor microenvironment (TME) of HPV-positive tumors (2). PD-L1 overexpression has been demonstrated in up to 85% of squamous cell carcinomas and 10-20% of adenocarcinomas. Immunosuppressive TME promotes the transition from dysplasia to invasive carcinoma (11). Some clinical trials investigated immune checkpoint inhibitors in metastatic, persistent or recurrent CC. The phase III studies EMPOWER-Cervical 1, KEYNOTE-826 and BEATcc demonstrated benefits of cemiplimab, pembrolizumab and atezolizumab in this setting in the first- and second-line setting (12-14). In the pivotal KEYNOTE-826 study, pembrolizumab was used in addition to chemotherapy, and bevacizumab could also be administered at the investigators’ discretion.
The randomized, double-blind phase III KEYNOTE-A18 trial tested pembrolizumab plus CRT followed by pembrolizumab maintenance therapy in locally advanced high-risk cancer. Patients with stage IB2-IIB (node-positive) or III-IVA (node-positive or -negative) according to FIGO 2014 participated. The study arm (n = 529) received cisplatin plus radiotherapy in combination with pembrolizumab 200 mg every three weeks for five cycles. Maintenance consisted of pembrolizumab 400 mg every six weeks for 15 cycles. In the control group (n = 531), placebo was used instead of pembrolizumab. PD-L1 positivity according to the combined positive score (CPS) was 94.9 % and 93.8 % respectively.
The addition of pembrolizumab resulted in a 30 % risk reduction with regard to the PFS defined as the primary endpoint (HR: 0.70; p = 0.002) (15). The positive outcome of the study was confirmed by the analysis presented at the ESMO Congress 2024: Compared to the control arm, the concomitant CRT/pembrolizumab strategy caused a significant reduction in mortality risk by 33% (HR: 0.67; p = 0.004; Figure. 1), with the benefit also being consistent in the subgroups with FIGO III and IVA (16).
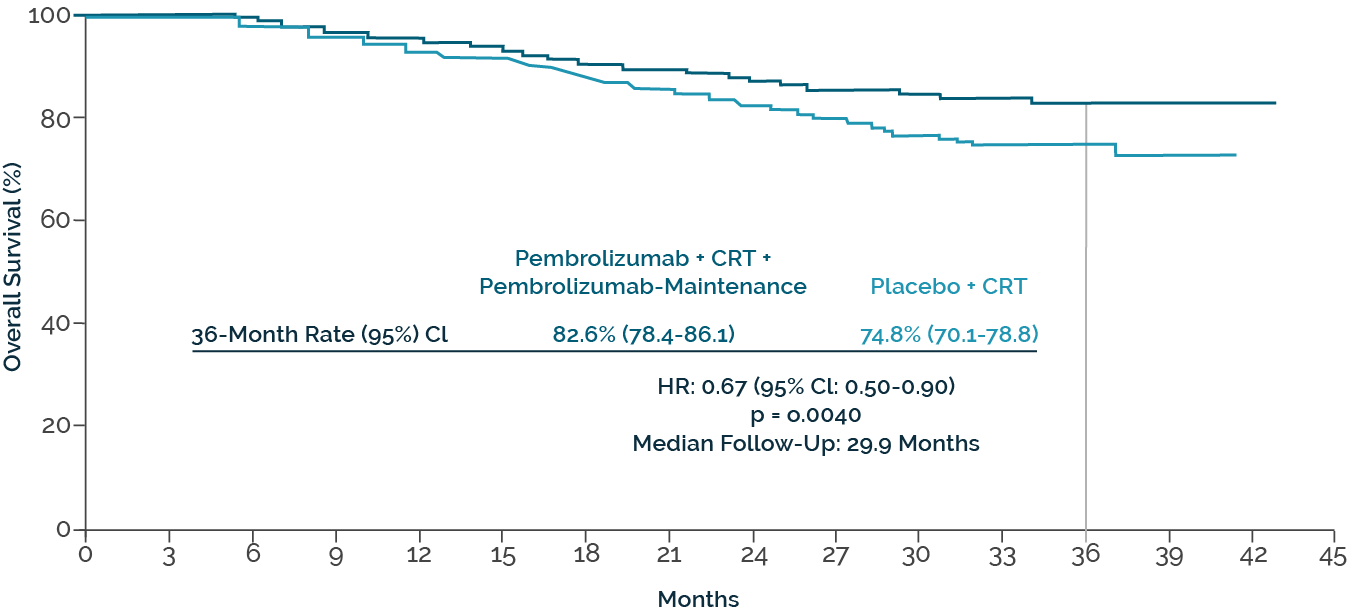
Fig. 1. Overall survival with pembrolizumab plus chemoradiotherapy vs. chemoradiotherapy in KEYNOTE-A18 (modified according to Lorusso D el al.)
Current Considerations And A Look Into The Future
Following the establishment of immunotherapy as a standard of care for metastatic CC, two pivotal phase III trials showed significant PFS and OS benefits in the treatment of LACC. Both ICT before CRT and CRT plus immuno-oncology therapies and immuno-oncology maintenance are currently considered primary treatment options globally. Factors such as approval, availability, the clinical and technical expertise of the teams and the characteristics of the healthcare systems will have a significant impact on the actual use of these therapies around the world and in Switzerland.
In metastatic CC, there has been a transition from the combination of chemotherapy and bevacizumab to chemotherapy and immunotherapy with or without bevacizumab. Sequencing and rechallenge of immunotherapy in first-line and subsequent lines are challenged by the use of checkpoint inhibitors as the primary treatment option for LACC. There is therefore an urgent need for new options.
Several early studies on the use of antibody-drug conjugates from the second-line onwards were presented at the ESMO Congress 2024. Tisotumab vedotin is not yet widely available, but a positive phase III study underpins the existing results (17). Promising data on trastuzumab deruxtecan and sacituzumab tirumotecan were also presented. Of particular relevance are the results for the combination of sacituzumab-tirumotecan and pembrolizumab, which showed an overall response rate of 57.9 % and an 82.1 % response rate at six months in relapsed or metastatic CC (18). Such advances are very welcome in this difficult-to-treat setting and give hope to patients in Switzerland and worldwide.
Explore more about the latest gynecological cancers with Drs. Apostolos Sarivalasis, Ilaria Colombo, and Christina Fotopoulou.
References
- H. Sung et al., “Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries,” CA a Cancer Journal for Clinicians, vol. 71, no. 3, pp. 209–249, Feb. 2021, doi: 10.3322/caac.21660.
- K. S. Okunade, “Human papillomavirus and cervical cancer,” Journal of Obstetrics and Gynaecology, vol. 40, no. 5, pp. 602–608, Sep. 2019, doi: 10.1080/01443615.2019.1634030.
- M. Arbyn et al., “Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis,” The Lancet Global Health, vol. 8, no. 2, pp. e191–e203, Dec. 2019, doi: 10.1016/s2214-109x(19)30482-6.
- WHO Classification of Tumours Editorial Board: 2020. 5th Edition, Volume 4
- A. B. Olawaiye, T. P. Baker, M. K. Washington, and D. G. Mutch, “The new (Version 9) American Joint Committee on Cancer tumor, node, metastasis staging for cervical cancer,” CA a Cancer Journal for Clinicians, vol. 71, no. 4, pp. 287–298, Mar. 2021, doi: 10.3322/caac.21663.
- M. Y. Salib et al., “2018 FIGO Staging Classification for Cervical Cancer: Added Benefits of Imaging,” Radiographics, vol. 40, no. 6, pp. 1807–1822, Sep. 2020, doi: 10.1148/rg.2020200013.
- “FIGO staging for carcinoma of the vulva, cervix, and corpus uteri,” International Journal of Gynecology & Obstetrics, vol. 125, no. 2, pp. 97–98, Feb. 2014, doi: 10.1016/j.ijgo.2014.02.003.
- “Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: A Systematic Review and Meta-Analysis of Individual patient data from 18 randomized trials,” Journal of Clinical Oncology, vol. 26, no. 35, pp. 5802–5812, Nov. 2008, doi: 10.1200/jco.2008.16.4368.
- McCormack M et al.: ESMO 2023; Abstr. #LBA8
- G. Eminowicz et al., “710MO Induction chemotherapy followed by chemoradiation in locally advanced cervical cancer: Quality of life outcomes of the GCIG INTERLACE trial,” Annals of Oncology, vol. 35, p. S545, Sep. 2024, doi: 10.1016/j.annonc.2024.08.773.
- S. J. Piersma, “Immunosuppressive tumor microenvironment in cervical cancer patients,” Cancer Microenvironment, vol. 4, no. 3, pp. 361–375, May 2011, doi: 10.1007/s12307-011-0066-7.
- K. S. Tewari et al., “Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240),” The Lancet, vol. 390, no. 10103, pp. 1654–1663, Jul. 2017, doi: 10.1016/s0140-6736(17)31607-0.
- N. Colombo et al., “Pembrolizumab for persistent, recurrent, or metastatic cervical cancer,” New England Journal of Medicine, vol. 385, no. 20, pp. 1856–1867, Sep. 2021, doi: 10.1056/nejmoa2112435.
- A. Oaknin et al., “Atezolizumab plus bevacizumab and chemotherapy for metastatic, persistent, or recurrent cervical cancer (BEATcc): a randomised, open-label, phase 3 trial,” The Lancet, vol. 403, no. 10421, pp. 31–43, Dec. 2023, doi: 10.1016/s0140-6736(23)02405-4.
- D. Lorusso et al., “Pembrolizumab plus Chemotherapy for advanced and recurrent cervical cancer: Final analysis according to Bevacizumab use in the randomized KEYNOTE-826 study,” Annals of Oncology, Oct. 2024, doi: 10.1016/j.annonc.2024.10.002.
- D. Lorusso et al., “793TiP A phase III, randomized, open-label, multicenter study of sacituzumab tirumotecan (sac-TMT) monotherapy vs treatment of physician’s choice chemotherapy in patients with endometrial cancer who have received prior chemotherapy and immunotherapy: ENGOT-en23/GOG-3095/MK-2870-005,” Annals of Oncology, vol. 35, p. S592, Sep. 2024, doi: 10.1016/j.annonc.2024.08.2153.
- I. Vergote et al., “Tisotumab vedotin as Second- or Third-Line therapy for recurrent cervical cancer,” New England Journal of Medicine, vol. 391, no. 1, pp. 44–55, Jul. 2024, doi: 10.1056/nejmoa2313811.
- J. Wang et al., “716MO Efficacy and safety of sacituzumab tirumotecan (sac-TMT) plus pembrolizumab in patients with recurrent or metastatic cervical cancer,” Annals of Oncology, vol. 35, pp. S548–S549, Sep. 2024, doi: 10.1016/j.annonc.2024.08.778.







