With an estimated 2.3 million cases diagnosed annually, breast cancer continues to be the most common cancer among women globally (1). Despite rising incidence rates, improvements in early detection and targeted therapy have caused mortality rates to decline. Among these developments, antibody-drug conjugates (ADCs) have become a game-changing therapeutic approach, especially for triple-negative breast cancer (TNBC) and hormone receptor (HR)-positive/HER2-negative breast cancer. Recently, Dr. Christian Kurzeder, Head of Breast Center at University Hospital Basel, and Dr. Marcus Vetter, Head of Hematology & Oncology at Cantonal Hospital Baselland, discussed the changing role of ADCs, their efficacy in clinical trials, and the management of associated adverse events.
Understanding Breast Cancer Subtypes
Breast cancer is a heterogeneous disease categorized into distinct subtypes based on molecular and histopathological characteristics (Fig. 1). These include luminal A, luminal B, HER2-enriched, and basal-like cancers, all of which exhibit characteristic gene expression profiles. Immunohistochemistry (IHC) is frequently utilized to distinguish between these subtypes and provide individualized treatment plans (2).
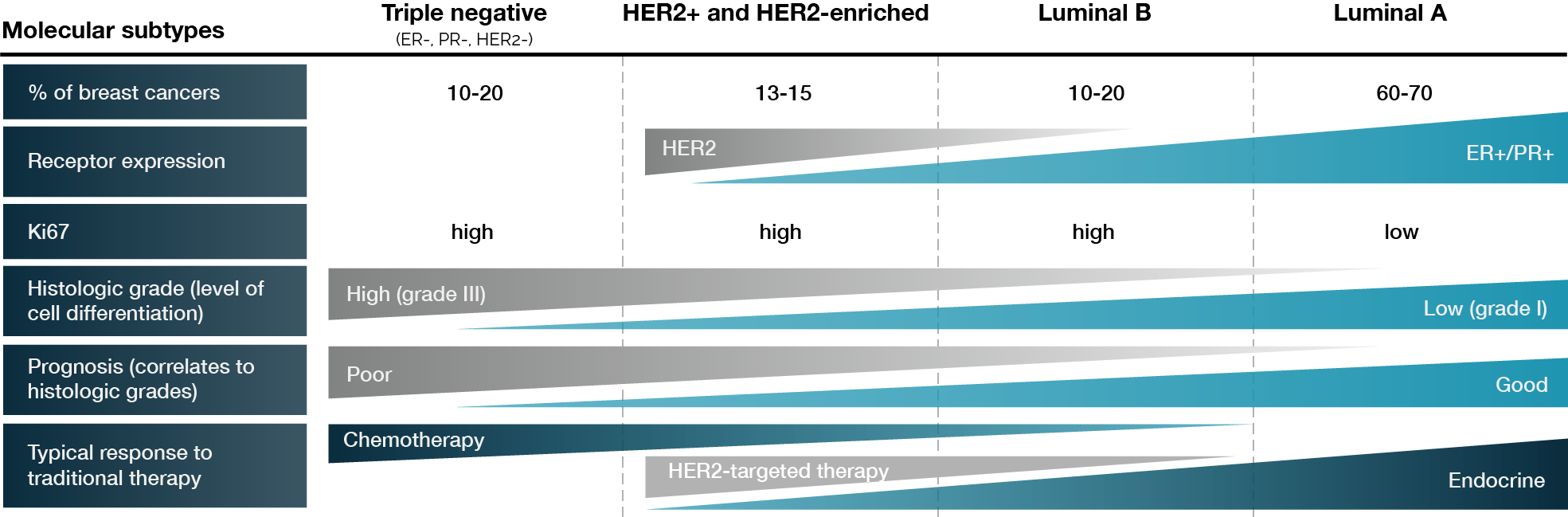
Figure 1. Molecular subtypes of breast cancer. Adapted from Wong E et al. (3).
There are already more than ten molecular therapeutic options for breast cancer, thanks to advancements in molecular biology that have enabled more tailored medicines. These therapies, which are increasingly customized according to genetic alterations in the tumor genome, serve as prognostic indicators, influencing both disease progression and treatment effectiveness.
In clinical practice, the estrogen receptor (ER), progesterone receptor (PR), HER2 expression, and proliferation markers such as Ki-67 are important indicators and targets for metastatic breast cancer. Treatment choices are also greatly influenced by BRCA mutations, PD-L1, PD-1, PIK3CA, ESR1, and changes in the AKT/mTOR pathway. These biomarkers refine therapeutic approaches and expand options for precision medicine.
An equally significant classification for choosing a course of treatment is the HER2 status. According to the ASCO/CAP guidelines, TNBC is defined by HER2 negativity and the absence of ER and PR expression. HER2 negativity indicates no HER2 overexpression or gene alteration. There are, however, HER2-low tumors within this category, which are characterized by measurable HER2 protein expression at low levels in the tissue (IHC 1+ or 2+ with in situ hybridization (ISH) negativity) (4).
Additionally, IHC and fluorescence-based tests such as ISH reveal that HER2 overexpression is apparent in HER2-positive and HER2-enriched breast tumors. The Ki-67 proliferation index further distinguishes luminal A and luminal B tumors, which are both defined by ER positivity (≥1%), with luminal B displaying a higher rate of cellular division. Furthermore, as poorer patient outcomes are associated with higher grades, histologic grading has a considerable impact on prognosis. Patients with TNBC experience the poorest outcomes, whereas those with high ER and PR expression generally have better prognoses. As a result, patient prognosis differs according to their molecular subtype. Similarly, HER2-enriched metastatic breast cancer has a more aggressive course (5).
HER2 and HR testing follow strict ASCO/CAP guidelines, integrating qualitative IHC assessments. In particular, IHC1+ denotes incomplete faint membrane staining, while IHC2+ indicates weak to moderate full membrane staining in over 10% of tumor cells. Additional ISH testing is necessary to validate the HER2 status in cases where IHC2+ is observed. The HER2-negative category encompasses tumors that are classified as IHC0, IHC1+, or IHC2+/ISH-negative; IHC1+ and IHC2+/ISH-negative may also be referred to as HER2-low tumors (4).
In hormone receptor (HR) testing, tumors that show estrogen receptor (ER) and progesterone receptor (PR) expression levels below 1% are classified as HR negative, whereas those with expression levels of 1% or higher are considered HR positive. Nevertheless, there remains an ongoing debate regarding the clinical implications of administering endocrine therapy to patients whose ER expression falls between 1% and 10% (6).
From an epidemiological perspective, luminal A and B subtypes constitute the majority of breast cancer cases, accounting for approximately 65%. Within this group, around 62% display HER2-low characteristics, defined as IHC1+ or IHC2+/ISH-negative. Triple-negative breast cancer (TNBC) represents about 15% of cases, with roughly 40% of these classified as HER2-low, while the remaining 60% are categorized as IHC0 (7).
Guidelines for ADCs in Breast Cancer Treatment
ADCs have significantly transformed the therapeutic approach to breast cancer, particularly in the metastatic setting. Current clinical guidelines, including those from ASCO, NCCN, and ESMO, recognize ADCs as essential treatment alternatives following the development of resistance to chemotherapy.
In the context of HR-positive/HER2-negative metastatic breast cancer, endocrine therapy continues to be the primary first-line treatment. Most patients receive several lines of endocrine-based therapies before developing resistance, at which point chemotherapy is generally considered. However, the efficacy of sequential single-agent chemotherapy is often limited, thereby positioning ADCs as a promising alternative. ADCs like sacituzumab govitecan and trastuzumab deruxtecan (T-DXd) have now been integrated into treatment guidelines as viable options when both endocrine therapy and chemotherapy fail.
Current treatment guidelines indicate that the initial approach for HR-positive, HER2-negative metastatic breast cancer typically involves the use of a CDK4/6 inhibitor in conjunction with endocrine therapy, unless there is an immediate risk of organ failure. For second-line therapy, the decision to continue with endocrine therapy, either alone or alongside targeted agents, is guided by molecular profiling. Patients facing imminent organ failure or those with rapidly advancing disease generally shift to chemotherapy or ADC-based regimens. Individuals identified as HER2-low may be treated with T-DXd, while sacituzumab govitecan is advised for HER2-zero patients after they have undergone two prior lines of chemotherapy (8).
With TNBC, which lacks specific hormonal and HER2-targeted therapies, ADCs have significantly transformed treatment strategies. The standard first-line treatment is contingent upon molecular profiling; PD-L1-positive patients are typically administered chemotherapy combined with checkpoint inhibitors, whereas those with BRCA mutations may respond favorably to PARP inhibitors. Nevertheless, ADCs have emerged as the preferred option for second-line treatment, with sacituzumab govitecan recommended following the failure of chemotherapy. For cases of HER2-low TNBC, T-DXd is considered a suitable third-line treatment alternative (8).
Overall, the integration of ADCs into treatment guidelines for both HR-positive/HER2-negative and triple-negative breast cancer underscores their efficacy and potential to improve patient outcomes, particularly in cases where traditional therapies fall short.
Key ADC Clinical Trials
ADCs facilitate the targeted delivery of higher doses of cytotoxic agents directly to the tumor microenvironment (9). For breast cancer, trastuzumab emtansine was the first ADC to receive approval in 2013. Since then, several other ADCs have been developed, including T-DXd and sacituzumab govitecan. A number of significant clinical trials have been conducted for these ADCs, particularly focusing on triple-negative and hormone receptor-positive, HER2-negative metastatic breast cancer.
ASCENT Trial
The ASCENT study demonstrated the efficacy of sacituzumab govitecan in metastatic TNBC (10). Patients included in the study had received at least two prior chemotherapies, one of which could be from the adjuvant setting if progression occurred within 12 months. A total of 529 patients were randomized to receive either sacituzumab govitecan or a treatment of the physician’s choice, including eribulin, gemcitabine, capecitabine, or vinorelbine. Patients with stable brain metastases were permitted but limited to a maximum of 15% of the study population.
The primary endpoint, progression-free survival (PFS), was assessed by independent review in the brain metastasis-negative population. The results were striking, showing a significant improvement in PFS from 1.7 to 5.6 months in favor of those receiving sacituzumab govitecan (Fig 2). Overall survival (OS) was nearly doubled, and the overall response rate was 35%, in contrast to just 5% in the chemotherapy group. This benefit was consistent across all HER2-negative subgroups, including those classified as HER2-low and IHC0.
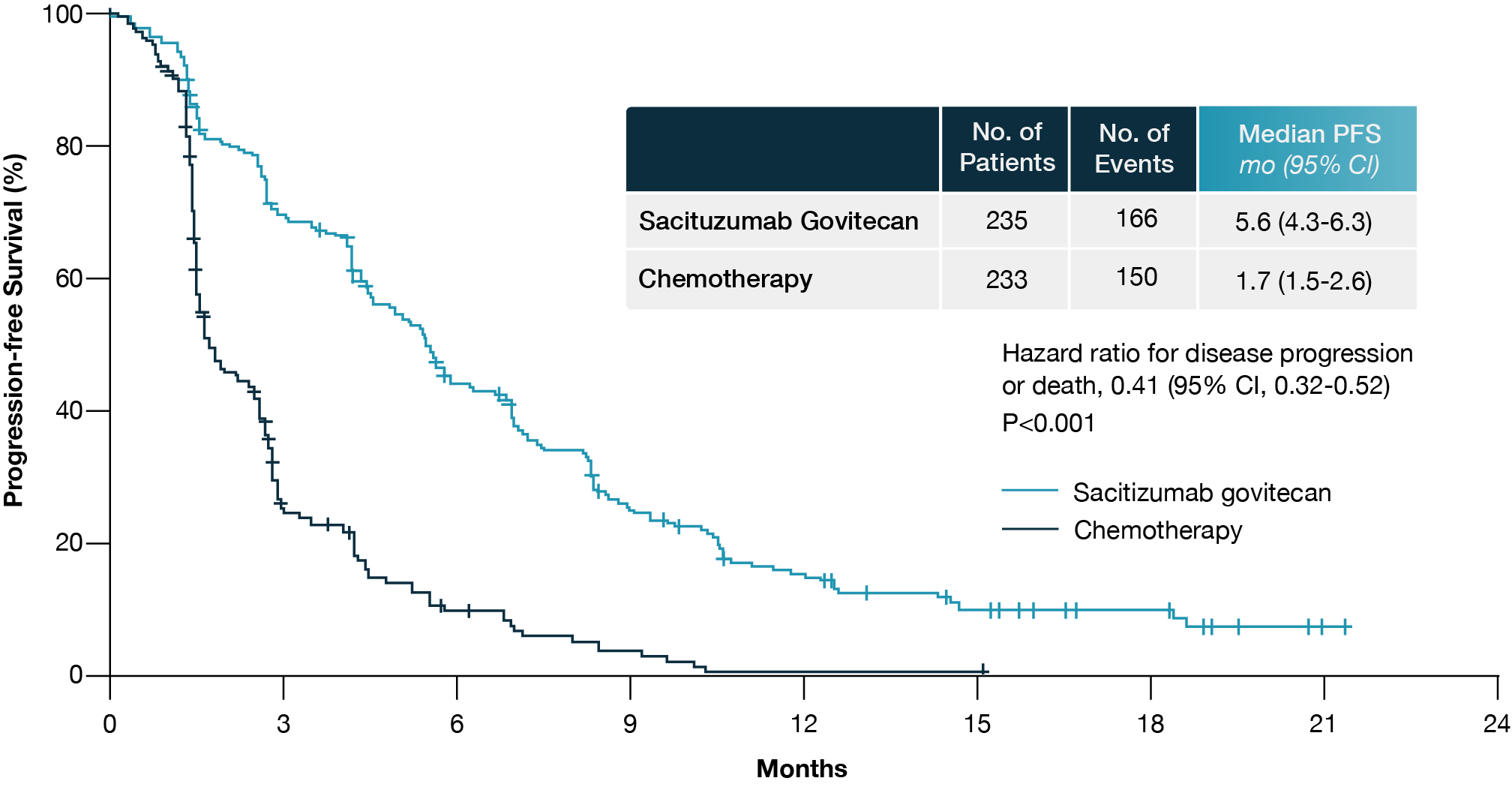
Figure 2. Progression-free survival in the brain metastasis-negative population of the ASCENT trial. Adapted from Aditya Bardia et al. (10).
TROPiCS-02 Trial
The TROPiCS-02 trial investigated the efficacy of sacituzumab govitecan in individuals diagnosed with HR-positive/HER2-negative metastatic breast cancer who had previously received at least one endocrine therapy, a taxane, and a CDK4/6 inhibitor (11). Patients had received a median of three prior chemotherapy regimens, with 95% exhibiting visceral metastases. A total of 543 patients were randomized to sacituzumab govitecan or a physician’s choice treatment (capecitabine, vinorelbine, gemcitabine, or eribulin).
The study demonstrated a statistically significant PFS benefit, with sacituzumab govitecan reducing the risk of progression or death by 34%. A higher proportion of patients remained progression-free at all landmark time points, with three times as many patients progression-free at one year. Additionally, OS benefit was significant, with median survival extending to 14.4 months in the sacituzumab govitecan group compared to 11.2 months for those receiving standard chemotherapy (12). These advantages were consistently observed across all subsets of HER2-negative patients, including those classified as HER2-low and IHC0.
DESTINY-Breast04 Trial
HER2-low breast cancer represents a newly recognized subset benefiting from HER2-targeted ADCs. The DESTINY-Breast04 trial enrolled patients with HER2-low, unresectable metastatic breast cancer who had received one to two prior lines of chemotherapy (13). Patients were randomized to receive T-DXd or physician’s choice treatment (capecitabine, eribulin, gemcitabine, or paclitaxel).
The study showed a clear improvement in median PFS in the HR-positive cohort, with a difference of 5.4 months favoring T-DXd. This benefit was also observed in the total study population, which included HR-negative patients. OS was notably prolonged, with a median difference exceeding 6 months in the HR-positive group (Fig 3).
In terms of safety, T-DXd presented a distinct toxicity profile compared to traditional chemotherapy, characterized by increased levels of nausea and fatigue while presenting lower incidences of neutropenia. Nonetheless, interstitial lung disease (ILD) or pneumonitis, a known risk associated with this drug, was observed in 12.1% of patients, highlighting the need for close monitoring and early intervention (13).
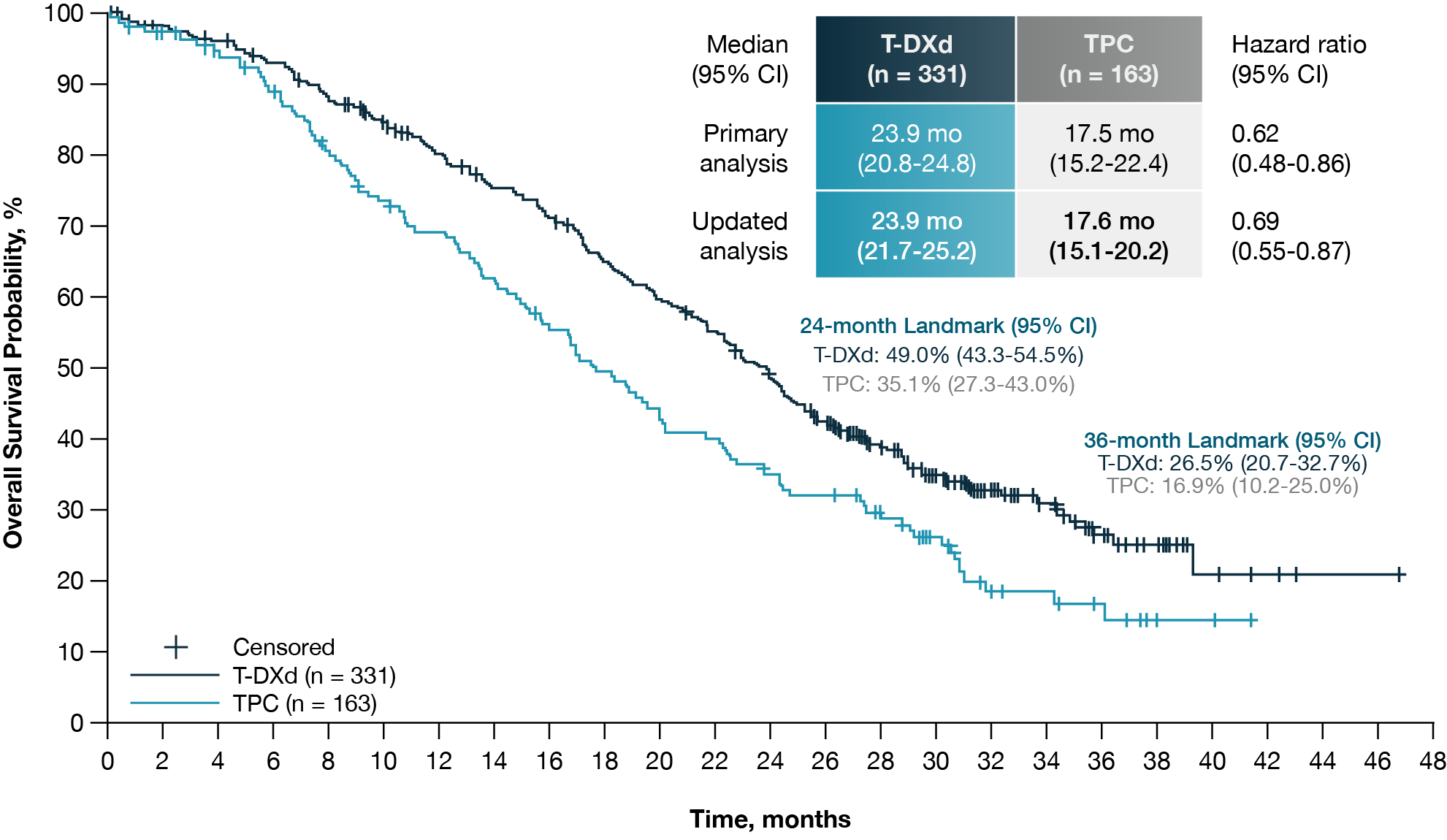
Figure 3. Updated overall survival in HR-positive, HER2-low unresectable, and/or metastatic breast cancer patients in the DESTINY-Breast04 Trial. Adapted from Shanu Modi et al (13).
Managing ADC-Associated Adverse Events
While ADCs demonstrate significant therapeutic efficacy, they also present distinct toxicities that necessitate proactive management approaches. The most frequently observed adverse events include hematologic and gastrointestinal toxicities, fatigue, and, in the case of T-DXd, ILD. A comprehensive understanding and management of these toxicities are crucial for enhancing treatment outcomes.
Diarrhea Management
Drs. Vetter and Kurzeder emphasize that while clinicians are familiar with managing diarrhea, patient education is key. Early-onset diarrhea may be attributed to cholinergic stimulation, while delayed-onset diarrhea is often linked to the cytotoxic payload. Patients should be educated to identify symptoms early and to initiate hydration and electrolyte replenishment promptly. First-line treatment includes antidiarrheal medications such as loperamide.
Assessing the severity of diarrhea is essential for determining the appropriate intervention. The standard protocol involves an initial dose of 4 mg of loperamide, followed by 2 mg after each loose stool, with a maximum daily limit of 16 mg. In instances of grade 3 or 4 diarrhea, treatment should be paused until symptoms resolve to grade 1 or below. Severe cases may necessitate hospitalization and correction of electrolyte imbalances. If high-grade diarrhea persists for more than three weeks without improvement, discontinuation of ADC therapy is advised (14).
Neutropenia Management
Neutropenia is a common toxicity, requiring close monitoring. If the absolute neutrophil count drops below 1,500/mm³ on day one or 1,000/mm³ on day eight, treatment should be paused. For grade 4 neutropenia lasting longer than seven days or in cases of febrile neutropenia, Drs. Vetter and Kurzeder suggest considering the use of granulocyte colony-stimulating factor (G-CSF) before implementing dose reductions. The administration of pegylated G-CSF after day eight can assist in sustaining neutrophil counts and minimizing treatment interruptions (14).
ILD with Trastuzumab Deruxtecan
ILD, though rare, is a potentially fatal complication of T-DXd. Drs. Vetter and Kurzeder emphasize the critical need for early identification, urging clinicians to monitor for symptoms such as dyspnea, persistent cough, fever, and unexpected fatigue. Any indication of ILD should trigger immediate cessation of treatment and a thorough diagnostic workup. In cases where ILD is suspected, the early administration of corticosteroids is essential to avoid the progression to severe respiratory failure (15).
Conclusion
The emergence of ADCs has revolutionized breast cancer treatment, offering targeted, highly effective options for patients with limited therapeutic alternatives. Clinical trials consistently demonstrate superior outcomes over conventional chemotherapy, particularly in TNBC and HR-positive/HER2-negative breast cancer. However, the risk of adverse events necessitates careful monitoring and proactive intervention to achieve the best possible patient outcomes
As research continues, ADCs are expected to play an increasingly prominent role in the evolving landscape of precision oncology, offering renewed hope for patients battling advanced breast cancer.
Watch the full e-learning course with Dr. Christian Kurzeder and Dr. Marcus Vetter to learn more about managing ADC-related adverse events in breast cancer.
References
- Melina Arnold et al., Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 66, 15–23 (2022).
- Emma Nolan et al., Deciphering breast cancer: from biology to the clinic. Cell 186, (8):1708–1728 (2023).
- Wong E. Breast cancer pathogenesis and histologic vs. molecular subtypes— Pathophysiology Review. https://www.pathophys.org/breast-cancer/breastcancer-copy/. Published December 29, 2012.
- Antonio C Wolff et al., Human Epidermal Growth Factor Receptor 2 Testing in breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused update. Arch Pathol Lab Med 142, (11):1364–1382 (2018).
- Yue Gong, et al. Impact of molecular subtypes on metastatic breast cancer patients: a SEER population-based study. Sci Rep 7, 45411 (2017).
- Kimberly H Allison et al., Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. JCO 38, 1346–1366 (2020).
- Paolo Tarantino, et al., Prognostic and biologic significance of ERBB2-Low expression in Early-Stage breast Cancer. JAMA Oncol 8, (8):1177–1183 (2022).
- ESMO Metastatic Breast Cancer Living Guidelines. Ann Oncol 32, (12):1475–1495 (2021).
- Zhiwen Fu, et al., Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Sig Transduct Target Ther 7, 93 (2022).
- Aditya Bardia et al., Sacituzumab govitecan in metastatic Triple-Negative breast Cancer. N Engl J Med 384, (16):1529–1541 (2021).
- Hope S. Rugo et al., Sacituzumab Govitecan in hormone Receptor–Positive/Human epidermal growth factor receptor 2–Negative metastatic breast cancer. JCO 40, 3365–3376 (2022).
- Hope S. Rugo et al., Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial. The Lancet 402, (10411):1423–1433 (2023).
- Shanu Modi, et al., Trastuzumab deruxtecan in previously treated HER2-Low advanced breast cancer. N Engl J Med 387, 9–20 (2022).
- Laura M. Spring et al., Sacituzumab Govitecan for Metastatic Triple-Negative breast cancer: Clinical overview and Management of potential toxicities. The Oncologist 26, (10):827–834 (2021).
- Sandra M. Swain et al., Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)–related interstitial lung disease/pneumonitis—Focus on proactive monitoring, diagnosis, and management. Cancer Treat. Rev. 106, 102378 (2022)








